Background and overview[1]
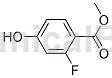
Methyl 2-fluoro-4-hydroxybenzoate can be used as a pharmaceutical synthesis intermediate. If 2-fluoro-4-hydroxybenzoic acid methyl ester is inhaled, please move the patient to fresh air; if skin contact occurs, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical treatment if you feel uncomfortable; If eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Structure
Preparation and Application[1]
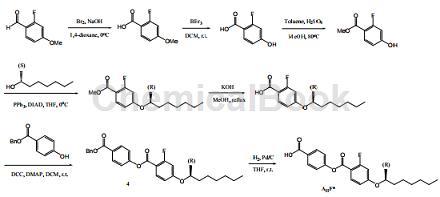
Methyl 2-fluoro-4-hydroxybenzoate can be used as a pharmaceutical synthesis intermediate. It can be prepared by 2-fluoro-4-methoxybenzaldehyde under the action of bromine and sodium hydroxide to generate 2-fluoro- 4-Methoxybenzoic acid, then removes the methyl group to generate 2-fluoro-4-hydroxybenzoic acid, and then reacts with methanol to form an ester to prepare 2-fluoro-4-hydroxybenzoic acid methyl ester.
Methyl 2-fluoro-4-hydroxybenzoate can be used as a pharmaceutical synthesis intermediate. For example, 2-fluoro-4-hydroxybenzoic acid methyl ester undergoes an esterification reaction with alcohol, and then removes the methyl group, and the carboxylic acid part forms an ester with an aryl alcohol. After a series of reactions, compound 4 can be generated, and then in hydrogen at room temperature Next, add compound 4 (10 g, 20 mmol) and catalyst 15% Pd/C (1.5 g) to a 500 ml round-bottomed double-neck flask, dissolve in THF (200 ml), and stir overnight. Remove the catalyst by passing through celite, filtering and washing with THF. The solvent was evaporated under reduced pressure to remove the crude oil, and the product was recrystallized with THF/n-hexane to obtain white solid AIIF* with a yield of 92%.
Main reference materials
[1] [Han C C, Chou Y C, Chen S Y, et al. Hydrogen-bonded bent-core blue phase liquid crystal complexes containing various molar ratios of proton acceptors and donors[J]. RSC Advances, 2016, 6( 38): 32319-32327.

 微信扫一扫打赏
微信扫一扫打赏

