background[1]
dibutyl phthalate (dbp) is a type of phthalate esters (paes) and is widely used in the plastics processing industry as a plasticizer. monobutyl phthalate (mbp) is a degradation product and synthetic intermediate of dbp. relevant studies have shown that mbp also has endocrine disrupting, carcinogenic, teratogenic and mutagenic properties. mbp can exist stably in nature, but it will accumulate and cause long-term harm to the environment.
detection method[1]
relevant investigations show that mbp can be detected in rivers, lakes, seas and garbage leachate. in particular, the concentration of mbp in garbage leachate is as high as 2500ug/l. therefore, the harm situation of mbp to the environment is becoming more and more severe, and it is of great significance to detect and safely control mbp content. currently, there are no standards, literature, or journals that provide detailed instructions for the detection of monobutyl phthalate content in consumer products. the testing method used in cn201711213210.9 can quickly and efficiently detect the content of monobutyl phthalate in textiles, achieving the purpose of monitoring and controlling monobutyl phthalate in textiles.
a method for detecting monobutyl phthalate in textiles, including the following steps:
(1) select a representative sample from the textile and use stainless steel scissors to cut the sample into small pieces;
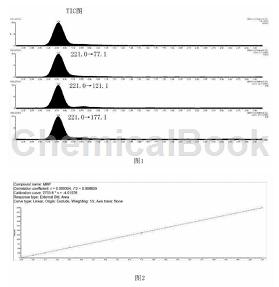
(2) weigh 1.0g of chopped sample, put it into a 40ml reaction tube, add 10ml of methanol, perform ultrasonic extraction in an ultrasonic cleaner, filter 1ml of the extraction solution with an organic filter membrane into a sample bottle, and use ultra-high efficiency the liquid chromatography tandem mass spectrometer analyzes the sample solution; the parameters of the ultra-high performance liquid chromatography tandem mass spectrometer are set as follows: instrument model, watersacquityhclass+xevotqd ultra-high performance liquid chromatography tandem mass spectrometer; chromatographic column model , watersbehc18 column, specifications: 50mm*2.1mm*1.7um; injection volume, 5 microliters; flow rate, 0.2ml/min; elution program, 40% water + 60% acetonitrile, hold for 3 minutes; acquisition mode, negative ion mrm mode ; capillary voltage, 1.5kv; desolvation gas flow rate, 650l/h; desolvation gas temperature, 350°c; parent ion 221.0, cone voltage 30v; product ions 77.1, collision energy 15v, 121.1, collision energy 15v, 177.1, collision energy 10v; quantitative ion pair 221.0→77.1; qualitative ion pair 221.0→121.1, 221.0→177.1.
compared with the existing technology, the present invention has the following advantages: the methanol has good wetting ability on textiles, and the monobutyl phthalate has good dissolving ability; at the same time, ultra-high performance liquid chromatography is used to as an analytical instrument, the tandem mass spectrometer is tested according to the instrument parameters set in this method. it can efficiently separate monobutyl phthalate from impurities and quickly and accurately characterize and quantify monobutyl phthalate. the high-performance liquid chromatography tandem mass spectrometer has a good linear range in the concentration range of monobutyl phthalate from 10ug/l to 10000ug/l. the linear correlation coefficient (r2) is greater than 0.995, and the method detection limit reaches 10ug/l. g, the rsd (relative standard deviation) obtained from the repeatability experiment is 1.79%-4.92%, the rsd obtained from the repeatability experiment is 2.62%, and the spike recovery rate is 90.7%-103.2%.
this method is simple, fast, accurate and reliable, and can meet the requirements for monitoring the content of monobutyl phthalate in textiles. figure 1 is a tic chart and an ion pair extraction chart of monobutyl phthalate of the present invention. figure 2 is a standard curve diagram of monobutyl phthalate of the present invention.
preparation[2]
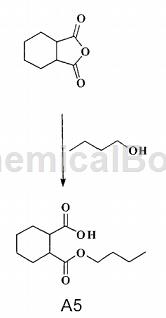
the dianhydride compound (1.5g, 10mmol) was dissolved in n-butanol (20ml), and heated to reflux for 4 hours. the n-butanol is evaporated under reduced pressure to obtain the first intermediate product a5 of the crude product, which is monobutyl phthalate.
apply[2]
can be used to synthesize cytarabine derivative 5. cytarabine is an analog of cytosine nucleoside and an inhibitor of dna polymerase. it can prevent dna synthesis, can also be incorporated into dna and interfere with dna replication. in addition, it can block the reduction of cytosine nucleotides into deoxycytosine nucleotides.
currently, cytarabine is mainly used for the treatment of acute leukemia. it has the best effect on acute myeloid leukemia, and is also effective on acute monocytic leukemia and acute lymphoblastic leukemia. it has certain effects on malignant lymphoma, lung cancer, digestive tract cancer, and head and neck cancer. it is also effective on viral keratitis and epidemic diseases. it also has certain effects on conjunctivitis, etc. however, it is not effective on most solid tumors. the activity of cytarabine is not very high. in order to improve the efficacy, cytarabine is generally combined with other drugs, such as: daunorubicin, all-trans retinoic acid combined with arsenic trioxide, pirarubicin, topotecan- etoposide-cyclophosphamide, fludarabine, etc. are used together. cytarabine has side effects such as bone marrow suppression and gastrointestinal reactions. a few patients may have side effects such as abnormal liver function, fever, and rash.
cytarabine is an anti-metabolite drug. it is first phosphorylated by deoxycytidine enzyme in cells and converted into active cytarabine, and then further converted into the corresponding diphosphate and triphosphate cytarabine. glycosides act. cytarabine mainly inhibits dna polymerase by competing with deoxycytidine triphosphate required in the dna synthesis process, interfering with the incorporation of nucleotides into dna. it can also inhibit nucleotide reductase and prevent the conversion of nucleotides into deoxygenase. nucleotide, but has no significant effect on the synthesis of rna and protein. it is a cell cycle-specific drug that acts on the s phase. it is most sensitive to cells in the s proliferation phase and is also effective on g1/s and s/g2 transition phases. effect. it disappears from the blood quickly after intravenous injection, and 40% can pass through the blood-brain barrier. the drug is mainly metabolized in the liver to inactive arabinoside, and 70% to 90% is excreted through the kidneys.
main reference materials
[1] cn201711213210.9 a method for detecting monobutyl phthalate in textiles
[2] faming zhuanli shenqing, 101787066, 28 jul 2010

 微信扫一扫打赏
微信扫一扫打赏

