Background and overview[1][2]
Atracurium besylate is a new benzylisoquinoline medium-effective non-depolarizing muscle relaxant. It is one of the 10 isomers of atracurium and has the same characteristics as atracurium. Similar muscle relaxant effects, metabolic processes and advantages of vecuronium. Atracurium besylate is a single isomer of atracurium besylate. It has a similar muscle relaxant effect and metabolism to atracurium besylate, but its muscle relaxant intensity is about that of atracurium besylate. It does not release histamine and has little cardiovascular reaction. It is an ideal medium-lasting non-depolarizing muscle relaxant.
Purpose[3]
Atracurium besylate is a symmetrical bisquaternary ammonium ester, which is a medium- and short-acting tubecura non-depolarizing muscle relaxant. It works by competitively binding to cholinergic receptors and can be reversed by anticholinesterase drugs such as neostigmine. It takes effect quickly and has good muscle relaxing effect. It can be used to relax skeletal muscles during various surgical anesthesia and facilitate breathing control. It is especially suitable for maintaining muscle relaxation during tracheal intubation and cesarean section. It can also be continuously infused to maintain long-term neuromuscular blockade.
Adverse reactions[4]
1. Rapid intravenous injection of large doses can cause hypotension, tachycardia and bronchospasm.
2. Some patients with allergies may release histamine, causing transient skin flushing.
Drug interactions[4]
1. This product should not be mixed with alkaline drugs such as thiopental sodium.
2. The muscle relaxant effect of atracurium can be antagonized by the cholinesterase inhibitor neostigmine.
3. This product can be used in combination with inhaled anesthetics, aminoglycosides and peptide antibiotics to enhance its muscle relaxant effect.
Preparation[1-2]
Method 1: (1R-cis, 1’R-cis)-2,2′-(3 , 11-dioxo-4,10-dioxotridedecylmethylene)bis(1,2,3,4,tetrahydro-6,7-dimethoxy-2-methyl-1-vertebrate Atracurium besylate (1R-trans, 1’R- trans)-5], yield 67%, content 99%.
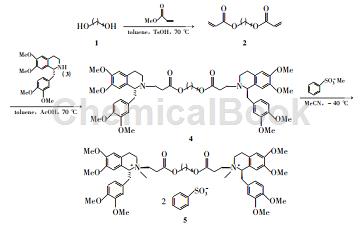
Step 1): 4Synthesis
Add 349.2g (143mmol) of toluene (140mL) solution into the reaction bottle, add 210.8g (51mmol) of toluene (50mL) solution and 3.5mL (61mmol) of acetic acid in sequence while stirring, and react at 70°C for 6 hours; reduce The solvent was evaporated under pressure and purified by silica gel-024-Synthetic Chemistry Vol.22, 2014 column chromatography (eluent: A=V (petroleum ether):V (ethyl acetate)=1:3) to obtain yellow oily liquid 441.4 g, yield 90%;
Step 2): Synthesis of atracurium besylate
Add 425.2g (28mmol) and 100mL of acetonitrile in sequence to the reaction bottle, stir to dissolve; add 15.2mL (112mmol) of methyl benzenesulfonate dropwise at -40°C, complete the drops, and react for 72h (TLC detection) [9-10], where (1R-trans, 1’R-trans)-5/(1R-cis, 1’R-trans)-5/(1R-cis, 1’R-cis)-5=70 /29/1. The solvent was evaporated to dryness and then subjected to silica gel column chromatography [Gradient eluent: V (ethyl acetate): V (methanol) = 5: 1 → V (dichloromethane): V (methanol) = 5: 1 (containing 0.05% Benzenesulfonic acid)] gradient elution, evaporate the eluate to dryness, dissolve it in 300 mL of methylene chloride, wash with water (50 mL) and 10% sodium chloride solution (50 mL), dry with anhydrous sodium sulfate, and concentrate to obtain a white solid Atracurium besylate 23.3g, yield 67%, content 99%
Method 2: A method for preparing atracurium besylate, specifically, it includes the following steps:
(1) Dissolve tetrahydropapaverine hydrochloride in water, adjust the pH value to about 10 with NaOH solution, extract the resulting mixture with toluene, dry it with anhydrous sodium sulfate, and concentrate to obtain product 1;
(2) Dissolve the product 1 in methanol, add N-acetyl-L-leucine for reflux reaction for 30 minutes, cool to room temperature, add diethyl ether, leave to crystallize, filter, and concentrate the filtrate to dryness to obtain white color Solid product 2;
(3) Dissolve the product 2 in water, adjust the pH value to about 10 with NaOH solution, add toluene for extraction, combine the toluene layers, and evaporate the solvent under reduced pressure to obtain the yellow oily product 3;
(4) The product 3.��Pentyl acrylate and glacial acetic acid are mixed, stirred and heated to react, after the reaction is completed, add toluene, stir and concentrate, pass the concentrate through a silica gel column, elute, collect the eluate from the first point, and concentrate to obtain light yellow oily product 4;
(5) Add methyl benzenesulfonate to the product 4 and stir at room temperature overnight. Add toluene and water to the reaction solution and stir for 10 minutes. Transfer the reaction solution to a separatory funnel and separate the water layer and the toluene layer. Wash with water, combine the aqueous layers, wash the aqueous layer with toluene, extract with dichloromethane three times, evaporate the solvent and add dropwise to anhydrous ether with stirring to precipitate the solid, filter, wash with anhydrous ether, and dry in a vacuum to obtain a white solid Product 5; and
(6) Perform silica gel column chromatography on the product 5, collect the eluate of the desired product points, evaporate the solvent, add an appropriate amount of water to dissolve, adjust the pH to 3.5 with benzenesulfonic acid, and freeze-dry to obtain a white solid of atracurium besylate.
Main reference materials
[1] CN201210446799.8 Atracurium for injection
[2] Improvement of the synthesis process of atracurium besylate
[3] Practical Drug Handbook
[4] New Clinical Pharmacology

 微信扫一扫打赏
微信扫一扫打赏

