Overview[1]
Polyphenylene ether is an aromatic polyether resin. Its chemical name is poly2.6-dimethyl-1.4-phenylene ether. It is made of 2.6-dimethylphenol under the action of a complex catalyst of copper salt and amine. Prepared by oxidative coupling polymerization. The polyphenylene ether molecular chain has high rigidity, excellent mechanical strength and high modulus, and outstanding creep resistance and water resistance. The glass transition temperature (Tg) is about 210℃, the upper limit of continuous use temperature is 120℃, the melting temperature is about 300℃, and decomposition occurs above 350℃. It is lightweight, non-toxic, has minimal water absorption, is flame retardant and self-extinguishing. The disadvantages are poor fluidity, stress cracking, and poor fatigue resistance. After being blended and modified with polystyrene polymers and reinforced with glass fiber, the above shortcomings can be greatly improved, and can be molded in general equipment by injection, extrusion and other methods. It is widely used as structural material and is an important variety of general engineering plastics. Polyphenylene ether and modified polyphenylene ether can be used in insulation materials, medical equipment, etc.
Structure
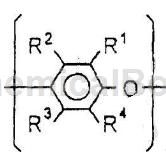
R1, R2, R3, R4 each independently represent H, alkyl group , substituted alkyl group, halogen atom, phenyl group or substituted phenyl group.
Performance[2]
(1) Physical and mechanical properties: The polyphenylene ether molecular chain contains a large number of benzene ring structures. The molecular chain has strong rigidity, high mechanical strength, high hardness and toughness; small creep and excellent dimensional stability.
(2) Thermal properties: Polyphenylene ether has high heat resistance, with a glass transition temperature of 211℃, a melting point of 268℃, and a thermal decomposition temperature of 330℃.
(3) Electrical properties: There are no strong polar groups in the molecular structure of polyphenylene ether. It can maintain good electrical properties in a wide temperature and frequency range. Its dielectric constant and dielectric loss tangent are It is the smallest among engineering plastics and is not affected by temperature, humidity and frequency.
(4) Chemical properties: Polyphenylene ether is an amorphous resin with no hydrolyzable groups in its molecular structure and good water resistance. The performance of the product will not change much after repeated use in high-pressure steam, but it can be dissolved in halogenated in aliphatic and aromatic hydrocarbons.
Modification[2]
1. Physical modification
1) Filling modification
Filling modification of materials can play a plasticizing role and reduce costs. In addition, it can also increase the Young’s modulus of the material and play a reinforcing role. The particle size and shape of the filler affect the performance of the material. After PPO modification, adding inorganic fillers can improve the tensile yield strength of modified polyphenylene ether (MPPO). According to literature reports, MPPO was filled and modified with three inorganic fillers: calcium carbonate (amorphous), talc (layered), and calcium silicate (needle-shaped). Calcium carbonate has no effect on the tensile yield strength of the filling system, while filling with talc and calcium silicate increases the tensile yield strength of the filling system. This is because calcium carbonate is a particle size filler. When the particle size of calcium carbonate is 2-10/xm, it only acts as an extender; when the particle size is less than 0.1/xm, it acts as a reinforcing agent.
2) Blending modification
(1) PPO/PS (polystyrene) alloy PPO and PS are both amorphous polymers with good compatibility and can form alloys in a wide range of PPO/PS blending ratios. Different blends The PPO/PS alloy has only one glass transition temperature (Tg). PPO/PS alloy can maintain the excellent characteristics of pure PPO and its fluidity is improved. (2) PPO/HIPS (high impact polystyrene)/elastomer alloy PPO has a relatively rigid molecular chain and is sensitive to notched impact strength. Adding HIPS can increase its notched impact strength, but not exceeding 15kJ/m2; for further To improve the notched impact strength of the system, add elastomer toughening modifiers. Currently, MPPO’s toughening modifiers mostly use SBS (styrene/butadiene/styrene copolymer) and EPDM (ethylene propylene terpolymer). Rubber), SEBS (hydrogenated styrene/butadiene/styrene copolymer) and other block elastomers. Among them, SEBS is the best. It not only improves the impact performance of MPPO, but also improves its aging resistance.
2. Chemical modification
1) End group modification
Terminal group modification is to perform an acylation reaction on the terminal hydroxyl group of PPO to convert the terminal hydroxyl group into -0c0c. Its original purpose is to prevent the terminal hydroxyl groups from being oxidized and improve the oxidation and thermal stability of PPO. End group modification includes esterification and etherification reactions. The end-capping method widely used in industry isA quinone coupling reaction is performed before the acylation reaction to reduce the formation of low molecular quinoid products. That is, under nitrogen and 5O60℃, the PPO reaction mixture is heated for 30 minutes, and then heated at 95℃ for 15 minutes, so that the PPO in the PPO reaction mixture combines with the quinoid by-product to form Quinone-coupled PPO, cool to 60℃, add water and toluene solution containing phase transfer catalyst, stir for 2 minutes, and then mix with vinegar
Acid anhydride undergoes acetylation reaction.
2) Main chain modification
A: Bromination and its derivative reactions
(I) Bromination reaction: PPO can undergo both electrophilic substitution and free radical reaction. At room temperature, a certain amount of Br2 solution is dropped into a 2% (mass fraction) PPO solution. Br2 mainly undergoes an electrophilic substitution reaction with the benzene ring of the main chain; when the Br2 concentration is low and the temperature is high, then It mainly reacts with the methyl group on the benzene ring. After the benzene ring is brominated, the amount of polymer increases significantly, and has a good linear relationship with the bromine content.
Methyl bromide only decreased. It shows that bromination of the benzene ring increases the rigidity of the chain, and methyl bromide increases the flexibility of the PPO chain.
(2) Phosphate esterification reaction: Methyl bromide PPO can undergo phosphorylation reaction. The phosphoric acid esterification of PPO is lower than that of pure PPO. This is due to the intramolecular plasticizing effect of the side chain phosphate ester groups. , reducing the intermolecular forces.
(3) Vinification reaction: Methyl bromide PPO can be converted into PPO with vinyl side chains through the action of a phase transfer catalyst. Under the action of an initiator, the MPPO and a specific cross-linking agent can produce lightweight Thermosetting composite materials have no small molecule volatiles, but due to the long reaction route and high production costs, industrialization is difficult.
B: Kylation and sulfonation: In tetrahydrofuran solvent, butyllithium is used as a catalyst, and PPO reacts with excess CO2 to carboxylate the methyl group on the benzene ring of PPO. Dissolve PPO in chloroform into a 10% PPO solution at room temperature, stir and slowly add fuming sulfuric acid dropwise, and react for 3-4 hours to obtain sulfonated PPO. The sulfonation reaction of PPO mainly occurs on the benzene ring.
Preparation[1]
A method for preparing polyphenylene ether, which method includes: contacting and mixing a polyphenylene ether solution polymerized in the presence of a non-water-soluble polymerization solvent and a catalyst with an aqueous solution of a chelating agent, terminating the polymerization reaction and Deactivate the catalyst; then add a water-soluble solvent of insoluble polyphenylene ether to precipitate the polyphenylene ether, and recover the precipitated polyphenylene ether; wherein (a) the polyphenylene ether solution is contacted and mixed with the chelating agent aqueous solution , kept at 50 to 120°C for 10 to 180 minutes; wherein (b) the mixture after separation and recovery of polyphenylene ether contains water-insoluble polymerization solvent and water-soluble poorly soluble polyphenylene ether Solvent, add this mixture to water to extract the solvent of the water-soluble and poorly soluble polyphenylene ether, so that the solvent of the water-soluble and poorly soluble polyphenylene ether is extracted into the water phase and separated from the polymerization solvent; wherein (c) by distillation The method separates the solvent of water-soluble and poorly soluble polyphenylene ether from the water phase, and circulates all or part of the remaining water for contact with the filtrate after the polyphenylene ether is separated, and the remaining high-boiling point organic matter in the water phase The content is 1% by weight or less.
Main reference materials
[1] Dictionary of Modern Materials Science and Engineering
[2] Modification and research progress of polyphenylene ether
[3] Preparation method of CN01129278.4 polyphenylene ether

 微信扫一扫打赏
微信扫一扫打赏

