Background and overview[1]
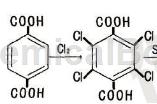
Tetrachloroterephthalic acid can be used as a pharmaceutical synthesis intermediate. If tetrachloroterephthalic acid is inhaled, move the patient to fresh air; if the skin comes in contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical treatment if you feel uncomfortable; if the eye contacts, remove the contaminated clothing. The eyelids should be separated, rinsed with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Apply[1-2]
Tetrachloroterephthalic acid can be used as a pharmaceutical synthesis intermediate. Examples of its application are as follows:
1) Preparation of dichlorsol, which belongs to the field of agricultural herbicide manufacturing. Dichlorol is a benzoic acid herbicide. It is a broad-spectrum herbicide used before emergence. In addition to treating pre-emergence grass, it can also be used for spraying on stems and leaves to remove annual grass weeds and some It is a broad-leaf weed that has good control effect on pre-emergent weeds such as crabgrass in drylands, and has significant activity on barnyard grass that has germinated to the second-leaf stage. The invention uses paraxylene, a bulk chemical raw material, as the main raw material, and uses the ammonia-oxygen method to produce terephthalonitrile; uses terephthalonitrile as the raw material through a gas-phase chlorination method to produce tetrachloroterephthalonitrile; and uses tetrachloroterephthalonitrile. Terephthalonitrile is used as raw material, and tetrachloroterephthalic acid is produced by solvent hydrolysis method; tetrachloroterephthalic acid is used as raw material, and the target product can be obtained in high yield by reacting with dimethyl sulfate in the solvent.
2) Used to improve the solubility of the third-generation quinolone broad-spectrum antibacterial drug ciprofloxacin. Ciprofloxacin and tetrachloroterephthalic acid were reacted under certain solvothermal conditions to successfully prepare a pharmaceutical co-crystal based on ciprofloxacin. The structure of the synthesized drug co-crystal was determined by X-ray single crystal diffraction as [ciprofloxacin][tetrachloroterephthalic acid]1.5. Its molecule contains one protonated ciprofloxacin and one undeprotonated ciprofloxacin. of tetrachloroterephthalic acid and half of deprotonated tetrachloroterephthalic acid. Because of the protonation of ciprofloxacin, this drug co-crystal retains the pharmacological properties of the drug itself while improving the water solubility of the raw drug and improving its bioavailability. The pharmaceutical cocrystal is prepared by a solvothermal method, which has high yield and good reproducibility. This method is simple and easy to implement and is conducive to industrial production.
Preparation[3]
Preparation of tetrachloroterephthalic acid by chlorination of terephthalic acid: Add an appropriate amount of terephthalic acid and iodine to a flask, add an appropriate amount of 40%-50% sulfuric acid, pass in chlorine gas in the dark, and keep the temperature at 80℃~90℃. In the later stage of the reaction, add sulfuric acid appropriately and the temperature can be raised to 130℃. After the ventilation is completed, it is lowered to room temperature. The material is slowly poured into ice water with stirring, and filtered under the temperature of ice water to obtain The white solid material, which is dried and crushed into powder form, is tetrachloroterephthalic acid.
Main reference materials
[1] CN201010295694.8 A new process for the production of diclozolin
[2] CN201710211329.6 The structure of a co-crystal of ciprofloxacin and tetrachloroterephthalic acid and its solvothermal preparation method
[3] CN200610038423.8 An industrial method for preparing tetrachloroterephthalonitrile

 微信扫一扫打赏
微信扫一扫打赏

