Background and overview[1]
4-Trifluoromethylphenylamidine hydrochloride is used as a pharmaceutical synthesis intermediate. If 4-trifluoromethylbenzimidine hydrochloride is inhaled, please move the patient to fresh air; if there is skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical treatment if you feel uncomfortable; If eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
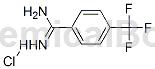
Structure
Preparation[1]
The preparation of 4-trifluoromethylbenzimidine hydrochloride is as follows: Weigh 85.5g (0.5mol) of 4-trifluoromethylbenzonitrile into a 250mL round-bottomed flask, add 200mL of methanol to dissolve it, and add the mass 5.6g (0.052mol) of methanol solution of sodium methoxide with a percentage of 50% was stirred magnetically at room temperature for 3 hours. After adding 27.5g (0.515mol) of ammonium chloride into the above system, the reaction was continued at room temperature for 48h. TLC tracks the end point of the reaction. After the reaction, the solvent is evaporated to dryness. The remaining solid is washed with diethyl ether, filtered, and repeated three times. The filter cake is sequentially recrystallized with hot water (about 60°C) and ethanol to obtain white needle-like crystals of 4-trifluoromethyl. 97g of benzamidine hydrochloride, yield 86.4%.
Application
4-Trifluoromethylphenylamidine hydrochloride is used as a pharmaceutical synthesis intermediate. For example: weigh 84.2g (0.375mol) of 4-trifluoromethylbenzimidine hydrochloride in a 500mL round-bottomed flask, add 250mL of water to dissolve, and add 280g of 10% mass percentage sodium hypochlorite aqueous solution under ice-water bath conditions. (0.376mol). After the dropwise addition is completed, gradually rise to room temperature, continue the reaction for 5 hours, stop stirring, filter, place the obtained filter cake in a 250mL round-bottomed flask, add about 50mL methanol to dissolve, and add 37.5g (0.375mol) dropwise under ice-water bath conditions. ) 100 mL of methanol solution of potassium thiocyanate. Potassium chloride precipitated out after about 30 minutes. Raise to room temperature and react for 12 hours. TLC tracks the end point of the reaction. After the reaction is completed, filter and evaporate the filtrate to dryness. The solid obtained is recrystallized with methanol and water (volume ratio 1:5) to obtain white granular crystals 3-(4-trifluoromethylphenyl)-5. -Amino-1,2,4-thiadiazole 40.8g, yield 44.4%.
Main reference materials
[1] CN201010107499.85-amino-1,2,4-thiadiazole compounds and preparation method thereof

 微信扫一扫打赏
微信扫一扫打赏

