Background and overview[1]
With the development and application of tyrosine kinase inhibitors (TKIs), the clinical outcomes of patients with chronic myelogenous leukemia (CML) and urban chromosome-positive acute lymphoblastic leukemia (Ph+ALL) have been significantly improved. Imatinib mesylate is the first first-generation TKIs approved for marketing in the world. It can specifically block the binding position of adenosine triphosphate (ATP) on ABL kinase, preventing tyrosine residues from phosphorylation, thus Inhibits BCR-ABL protein activity, resulting in inhibited proliferation and increased apoptosis of BCR-ABL positive cells. This molecularly targeted therapy targeting pathogenesis has dramatically changed the prognosis of patients with CML and Ph+ ALL. However, nearly 35% of CML patients who receive imatinib do not achieve complete cytogenetic response (CCyR), which may be the main reason for the relapse of these patients. Second-generation TKIs (such as dasatinib and nilotinib) can improve the efficacy and prolong overall survival of patients who fail imatinib treatment. Currently, dasatinib and nilotinib have been approved as first-line treatments for patients in the chronic phase of CML. Although first- and second-generation TKIs have improved the clinical outcomes of CML and Ph+ ALL patients, resistance still occurs in some patients with BCRABL mutations, especially the T315I mutation. Prior to the approval of ponatinib (ponatinib), there were no TKIs on the market that could overcome drug resistance, refractory, or intolerance in patients with these BCR-ABL mutations. In response to the T315I mutation, ARIAD Pharmaceuticals developed the third-generation TKIs—ponatinib (Platinib). The unique mechanism of action of ponatinib (Platinib) that binds to the BCRABL kinase site can inhibit the activity of BCR-ABL kinase containing T315I mutations, bringing new hope to patients with CML and Ph+ ALL. Ponatinib (Platinib) was formerly known as AP24534, and its chemical name is 3-(2-imidazole[1,2-b]pyridazine-3-ethynyl)-4-methyl-N-[4- [(4-Methyl-1-piperazinyl)-methyl]-3-(trifluoromethyl)benzene]benzamide, relative molecular mass: 532.56, molecular formula: C29H27F3N6O.
Mechanism of action[1]
Ponatinib (Platinib) is an oral multi-target TKIs compound and a pan-BCR-ABL inhibitor. Its half-inhibitory concentration (IC50) for inhibiting the tyrosine kinase activity of ABL and T315I mutant ABL in vitro is 0.4 and 2.0 nmol·L-1 respectively, and the IC50 value is between 0.1 and 20 nmol·L-1. It also inhibits the activity of other oncogenic kinases. Including vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), erythropoietin human hepatocyte (Eph) receptor, cytoplasmic tyrosine kinase Src family (SFK), membrane receptor tyrosine kinase gene (C-kit), orphan receptor tyrosine kinase (RET), angiopoietin type I tyrosine kinase receptor 2 (TIE2) and FMS-like tyrosine kinase 3 (FLT3). The molecular structure of ponatinib (Platinib) is designed based on different ways of interacting with T315I and has nothing to do with the structure of existing kinase inhibitors. ABL inhibitors include aspartate-phenylalanine-glycine (DFG-in, class 1 inhibitor) and aspartate-phenylalanine-glycine-free (DFG-out, class 2 inhibitor) Two types, depending on how they bind and interact with BCR-ABL. Type 1 inhibitors are the first generation of small molecule kinase inhibitors, targeting the ATP binding site of the enzyme activation group and characterized by an open configuration of the activation ring; Type 2 inhibitors bind to other hydrophobic sites and bind to Type 1 inhibitors are different and form a configuration that is inactive. In contrast, class 2 inhibitors have higher selectivity and efficacy than class 1 inhibitors.
Class 1 inhibitors include sunitinib and dasatinib, and class 2 inhibitors include imatinib, nilotinib, and ponatinib (platinib). Regardless of the binding method, imatinib, dasatinib and nilotinib will form a key hydrogen bond with the side chain of Thr315 of the wild-type ABL gating gene residue, which is also partially lipophilic and hydrophobic near Thr315. bag combination. However, this hydrogen bond is easily damaged, and mutation of the gated gene residue to isoleucine also causes steric hindrance, limiting the inhibitor from binding to the adjacent Thr315 hydrophobic pocket. Therefore, imatinib, dasatinib, and nilotinib cannot inhibit ABL kinase activity with Thr315 point mutations. Clinically, T315I mutation has been observed in 15% to 20% of patients. This highly drug-resistant mutation occurs in the gate gene region of the ATP binding site of the BCR-ABL fusion protein ABL kinase. It is worth noting that ponatinib (Platinib) can avoid binding to the side chain of wild-type BCRABLT315I, forming a van der Waals attraction that is conducive to binding to the isoleucine in the side chain of the T315I mutant. In addition, ponatinib (platinib) can also regulate the steric hindrance of isoleucine mutants and inhibit ABL kinase activity in combination with the T315I point mutation.
In vitro studies have shown that ponatinib (Platinib) has a significant inhibitory effect on the proliferation of both sensitive and drug-resistant CML cell lines, and is effective regardless of wild-type BCR-ABL or T315I mutation. In addition, ponatinib (Platinib) also has anti-imatinib resistance mutants (KIT and PDGFRα) and anti-FGFR1-4, FLT3 activity [FGFR1-4, FLT3 and myelodysplastic syndromes] (MDS), acute myeloid leukemia (AML) and other malignant hematological tumors, it can effectively prevent the ectopic expression of T cell kinase Lck (Lck is a proto-oncogene, and ectopic expression in cells can induce cell carcinogenesis), but has little effect on endogenous Fyn and Src.
Clinical research[1]
1. Anti-CML
The inhibitory effect of imatinib on the tyrosine kinase activity of BCR-ABL fusion protein is used in the treatment of many CML patients.The growth and development of the cell population in the medium, especially the number of FGFR1-positive cell populations, was significantly reduced. It is suggested that for patients with refractory MDS8p11, ponatinib (platinib) is a very promising drug for their treatment and improvement of prognosis.
5. Stem cell leukemia/lymphoma syndrome (SCLL)
SCLL is a malignant proliferation disease of myeloid and lymphoid cells. Its onset is often related to the abnormal expression of FGFR1. It is characterized by sustained FGFR1 kinase activity and can quickly transform into AML and lymphoma, with extremely poor prognosis. At present, molecular targeted therapy has not been widely used in the treatment of SCLL. Ponatinib (Platinib), as a new generation of BCR-ABL inhibitor, also acts on the FGFR family. In the study of mouse BaF3 cells by REN et al. [27], it was found that the drug can effectively inhibit the phosphorylation of FGFR1 fusion kinase and its downstream effectors (such as PLCY, Stat5, Scr, etc.), and specifically inhibit mosaic FGFR1 fusion. Kinase CD34+ progenitor cells’ cell growth and colony formation, and significantly prolong the survival of SCLL cell line tumor-forming mice. A dose of 30 mg·kg-1 can also significantly delay or even inhibit tumor regeneration of mouse KG1 cells. These results suggest that the inhibitory effect of ponatinib (platinib) on FGFR1 fusion kinase is likely to be beneficial in the treatment of SCLL patients and other human diseases related to abnormal expression and activation of FGFR1.
6. Resistance to other malignant tumors of FGFR origin
Ponatinib (Platinib) is a multi-target kinase inhibitor. In recent years, multiple studies have found that it can powerfully inhibit the activity of FGFR1-4 family fusion kinases. In the experiment of inducing FGFR1-4 kinase expression and activation in mouse Ba/F3 cells, ponatinib (Platinib) (IC50<40nmol·L-1) effectively inhibited the FGFR-mediated signaling pathway. In the study of 14 cell lines represented by endometrial cancer, bladder cancer, gastric cancer, breast cancer, lung cancer, and colon cancer (including abnormal expression of FGFRs caused by various mechanisms), ponatinib (Platinib) (IC50 (7 to 181 nmol·L-1) can also inhibit the growth of tumor cells by inhibiting the FGFR-mediated signal regulatory pathway. Taking patients with endometrial cancer as the research subjects, it was found that the combination of ponatinib (platinib) and the mammalian rapamycin (mTOR) inhibitor ridaforalimus can inhibit FGFR2 and mTOR at the same time, exerting a dual pathway regulatory effect, thus Inhibits tumor growth, which provides a new treatment strategy for patients with high-risk endometrial cancer. The above results suggest that ponatinib (platinib) is an effective FGFR inhibitor and can be considered for the treatment of malignant neoplastic diseases originating from FGFR.
Preparation[1]
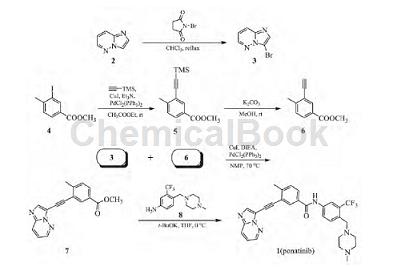
1) Preparation of 3-bromoimidazo[1,2-b]pyridazine (3)
Dissolve 200g (1.68mol) imidazo[1,2-b]pyridazine (2) in 2L chloroform, add 328.8g (1.85mol) N-bromosuccinimide, and heat to reflux (61 ℃) overnight, TLC showed that the reaction was complete. The organic phase was washed with water (1L×2), washed with saturated sodium chloride solution (1L×1), dried over anhydrous sodium sulfate, and spin-dried to obtain 321g of light yellow solid 3, yield 96.7%, mp145~147°C (Literature: mp146~147℃). MSm/z: 197.9[M+H]+.
2) Preparation of methyl 4-methyl-3-trimethylsilylethynylbenzoate (5)
Add 300g (1.09mol) of 3-iodo-4-methylbenzoic acid methyl ester (4), 2.06g of iodide (11mmol), and bis(triphenylphosphine)dichloride into a 5L three-neck flask. 7.63g of palladium (11mmol), 4L of ethyl acetate, 329.3g of triethylamine (3.25mol), 159.8g of trimethylsilyl acetylene (1.63mol) were introduced into the system to replace the gas in the system with argon gas, and the reaction system was closed at room temperature. Stir overnight and check that the reaction is complete by TLC. Filter the reaction solution, wash the filtrate with water (1L×2) and saturated sodium chloride solution (1L×1), dry with anhydrous sodium sulfate, and spin dry the solvent to obtain 267.4g of red solid 5, with a yield of 99%. It can be used directly without purification. Next reaction.
3) Preparation of methyl 3-ethynyl-4-methylbenzoate (6)
In a 5L three-neck flask, add 267.4g (1.09mol) compound 5, then add 4L methanol, stir mechanically to dissolve, add 225g (1.63mol) potassium carbonate, and monitor the reaction with TLC while mechanically stirring at room temperature (each time 1 minute) process, the reaction is completed in about 5 to 10 minutes. Pour the reaction solution into 5 L of water, stir evenly, filter the precipitate, wash once with 3 L of water, and dry the solid in vacuum to obtain 180 g of brown solid 6, yield 95%, mp 62~64°C.
4) Preparation of methyl 3-(imidazole[1,2-b]pyridazine-3-ethynyl)-4-methylbenzoate (7)
Add 180g (1.03mol) compound 6, 198g (1.01mol) compound 3, 1.9g (10mmol) copper iodide, and 7g (10mmol) bis(triphenylphosphine) dichloride into a 5L three-neck flask. Palladium, 3LN-methylpyrrolidone (NMP), 193.4g (1.50mol) diisopropylethylamine (DIEA) were introduced into the system to replace the gas in the system with argon, and mechanically stirred at 70°C overnight. TLC detected that the reaction was complete. Pour the reaction solution into 12L of water, stir evenly, and filter. After drying the solid obtained, add 1L of ethyl acetate and stir for 1 hour. Filter, wash the filter cake with ethyl acetate (200mL 185g of light yellow crystal 7 was obtained, yield 63.6%, mp 141~143°C.
5) Synthesis of ponatinib (Platinib) (1)
In a 2L three-neck flask, add 50g (0.18mol) 4-((4-methylpiperazin-1-yl)methylene)-3-trifluoromethylaniline (8), 53.3g ( 0.18mol) Compound 7, 500mL tetrahydrofuran, mechanically stirred, cooled to 0°C, slowly added dropwise under mechanical stirring conditions to a solution of 123g (1.10mol) potassium tert-butoxide dissolved in 500mL anhydrous THF, after the dropwise addition Maintain low temperature for 10 min, move to room temperature, stir overnight, and check that the reaction is complete by TLC. Pour the reaction solution into 3L of water, stir evenly, extract with ethyl acetate (1L×3), combine the organic phases, wash with water (1L×3), dry over anhydrous sodium sulfate, spin dry the solvent to obtain a yellow solid, add 100mL of acetic acid The solid was dissolved in ethyl ester and stirred for 2 h. Filter, dry, and recrystallize with 1,4-dioxane-water (volume ratio 1:1) to obtain 73g of light yellow solid ponatinib (platinib) (1), yield 71.9%, mp195 ~197℃. MSm/z: 533.3[M+H]+. The purity detected by HPLC (area normalization method) was 99.65%.
Main reference materials
[1] The third generation BCR-ABL tyrosine kinase inhibitor ponatinib (Platinib)
[2] Synthesis of new anti-leukemia drug ponatinib (Platinib)
� Dissolve in 500 mL of anhydrous THF solution. After the dropwise addition, keep the temperature low for 10 minutes, move to room temperature, and stir overnight. TLC detects that the reaction is complete. Pour the reaction solution into 3L of water, stir evenly, extract with ethyl acetate (1L×3), combine the organic phases, wash with water (1L×3), dry over anhydrous sodium sulfate, spin dry the solvent to obtain a yellow solid, add 100mL of acetic acid The solid was dissolved in ethyl ester and stirred for 2 h. Filter, dry, and recrystallize with 1,4-dioxane-water (volume ratio 1:1) to obtain 73g of light yellow solid ponatinib (platinib) (1), yield 71.9%, mp195 ~197℃. MSm/z: 533.3[M+H]+. The purity detected by HPLC (area normalization method) was 99.65%.
Main reference materials
[1] The third generation BCR-ABL tyrosine kinase inhibitor ponatinib (Platinib)
[2] Synthesis of new anti-leukemia drug ponatinib (Platinib)

 微信扫一扫打赏
微信扫一扫打赏

