Overview[1][2]
O-Toluenediamine is a light gray solid at room temperature and has an amine smell. It is an intermediate in the production of methylbenzotriazole, which is widely used. Methylbenzotriazole can be used as a metal rust inhibitor and corrosion inhibitor, an anti-tarnish agent for copper and silver, and a paint additive.
Purification[1-2]
The purity of commercially available o-toluenediamine raw materials is generally 70-90% by weight, which contains a small amount of m-toluenediamine, monoamine and other impurities. If these impurities are not removed and commercially available raw materials are reacted directly with sodium nitrite, these impurities will also react with sodium nitrite to produce dark-colored by-products. These colored impurities are difficult to separate and cannot be completely removed after multiple activated carbon decolorizations, making it difficult for the product to meet product quality requirements.
1. CN02111089.1 reports adding 0.05-0.5% by weight of salts of zinc, iron, magnesium, and copper to commercially available o-toluenediamine raw materials, and then performing conventional vacuum distillation.
In a 500 ml three-neck flask equipped with a distillation tower with a plate number of 20-22 and a thermometer, add 400 grams of o-toluenediamine raw material (purchased from Cangzhou Dahua Group Co., Ltd., measured by the internal standard method Purity: 85.5% by weight) and 0.4 g of zinc carbonate, vacuum distilled. The reflux ratio at the beginning was adjusted to about 10:1. When the liquid temperature was heated to 150-155°C, the front fraction of 76-118°C/650-800Pa was collected, totaling 3.4 grams. Then adjust the reflux ratio to about 5:1, and under the same liquid temperature conditions, collect the o-toluenediamine fraction of 118-120°C/650-800Pa, a total of 390.5 grams, and the residue at the bottom of the bottle is 5.5 grams. The purity of the o-toluenediamine fraction obtained was 99.6% (measured by normalization method).
2. CN201210248916.
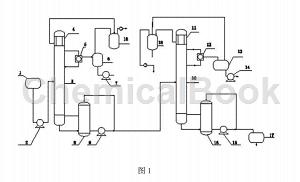
1) Send the crude o-toluenediamine into the storage tank (1), heat it to 100~120 ℃, and transport it through the pump (2) into the removal tower (3);
2) Under vacuum, the steam at the top of the tower (3) is condensed through the condenser (4), and the reflux volume is controlled by the reflux ratio controller (5), and part of the front fraction is sent to the storage tank (6) ), and transferred out by the pump (7), the liquid phase flowing back into the tower is distributed through the distributor, and heat exchanges with the gas phase to achieve gas-liquid balance; the material at the bottom of the tower is heated by the reboiler (8), and the gas phase material returns to the degassing In the low tower (3), high boiling point materials that cannot be gasified are transported to the product tower (10) through the pump (9);
3) Under the action of vacuum, the steam at the top of the product tower (10) is condensed through the condenser (11), and the reflux volume in the tower is controlled by the reflux ratio controller (12), and the product o-toluenediamine is sent to the storage tank. (13), and finally sent to the finished product storage tank through the pump (14). The liquid that flows back into the tower distributes the materials through the distributor and performs heat exchange with the gas phase to achieve gas-liquid balance. The liquid that flows back to the bottom passes through the pump (15) ) cycle, heated by the reboiler (16), and separated step by step, so that the qualified o-toluenediamine flows out from the top of the tower, and the high-boiling substance m-phenylenediamine is transported to the rear fraction storage tank (17) through the pump (15) )store.
The advantage of the present invention is that it uses two towers in series for rectification, which can continuously separate o-phenylenediamine, m-toluenediamine, monoamine and other impurities. After rectification, the purity of o-phenylenediamine reaches 99.5 %above.
Figure 1 is a schematic process flow diagram of the continuous purification and refining of o-toluenediamine according to the present invention.
As shown in Figure 1, 1. Storage tank; 2. Pump; 3. Stripping tower; 4. Condenser; 5. Reflux ratio controller; 6. Storage tank; 7. Pump; 8. Reboiler ; 9. Pump; 10. Product tower pump; 11. Condenser; 12. Reflux ratio controller; 13. Storage tank; 14. Pump; 15. Pump; 16. Reboiler; 17. Pump; 18. Vacuum tank .
Apply[1]
Sodium methylbenzotriazole can be made from the reaction of o-toluenediamine and sodium nitrite. Sodium methylbenzotriazole can be used as metal rust inhibitor and corrosion inhibitor, copper and silver anti-tarnish agent, coating additive, lubricating oil additive, etc. For example, page 718 of the World Handbook of Fine Chemicals (Continued) (edited by the Institute of Science and Technology Information of the Ministry of Chemical Industry, published by the Coal Industry Press, May 1986) introduces that sodium methylbenzotriazole has properties related to copper and copper alloys. Commonly used benzotriazole sodium has the same corrosion inhibition effect in acidic solutions.Or even better. Moreover, due to the utilization of industrial by-products, the production cost of sodium methylbenzotriazole is only about half of that of sodium benzotriazole.
The method of synthesizing sodium methylbenzotriazole through the reaction of o-toluenediamine and sodium nitrite has been reported. For example, U.S. Patent 4,158,660 and Japanese Patent Publication No. 04,360,878 describe a method of preparing sodium methylbenzotriazole by reacting o-toluenediamine with sodium nitrite in an acetic acid solvent and under normal pressure conditions. U.S. Patent 4,363,914 and Dalian Chemical Industry No. 3, 4 (joint issue) page 12 (1994) describe a method for preparing sodium methylbenzotriazole by reacting o-toluenediamine with sodium nitrite in water and under high pressure. Compared with the normal pressure method, the high pressure method does not require acetic acid, has fewer side reactions, less pollution, and a high yield.
Main reference materials
[1] CN02111089.1 Purification method of o-toluenediamine
[2] CN201210248916.X A method for continuously purifying and refining o-toluenediamine

 微信扫一扫打赏
微信扫一扫打赏

