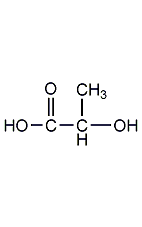
Structural formula
| Business number | 0139 |
|---|---|
| Molecular formula | C3H6O3 |
| Molecular weight | 90.08 |
| label |
DL-2-hydroxypropionic acid, Propanol acid, alpha-hydroxypropionic acid, DL-2-Hydroxypropanoic acid, Racemic lactic acid, a-Hydroxypropionic acid, sour agent, fungicides, preservative, antifungal agent, mordant, deliming agent, acidic solvent |
Numbering system
CAS number:50-21-5
MDL number:MFCD00004520
EINECS number:200-018-0
RTECS number:OD2800000
BRN number:1209341
PubChem number:24901202
Physical property data
1. Properties: The pure product is a colorless or slightly yellow viscous liquid with a sour milk smell and strong hygroscopicity.
2. Density (g/mL, 25/4℃): 1.209
3. Melting point (ºC, DL-lactic acid): 17~18
4. Melting point ( ºC, D-, L-lactic acid): 52.8
5. Boiling point (ºC, normal pressure): 122 (2kpa)
6. Refractive index (20ºC): 1.4351
7. Flash point (ºC): >110
8. Specific rotation (º): -0.05 º (c= neat 25 ºC)
9. Heat of combustion (KJ/mol, 25ºC): 1368.3
10. Solubility: Can be mixed with water, ethanol, and glycerin at will, soluble in ether, and insoluble in chloroform, petroleum ether and carbon disulfide.
Toxicological data
1. Skin/eye irritation: rabbit, skin contact, standard Draize test: 5mg? 24H, strong reaction; rabbit, eye contact, standard Draize test: 750ug, strong reaction 2. Acute toxicity: rat oral LD50: 3543mg/ kg; mouse oral LC50: 4875mg/kg; rabbit oral LDLo: 5mg/kg; rabbit skin contact LD50: >2mg/kg; rabbit rectal LD50: 600mg/kg; guinea pig oral LD50: 1810mg/kg; quail Oral LD50: >2250mg/kg3. Mutagenicity: Mutant microorganism detection system: Bacteria – Escherichia coli: 210ppm/3H4. It is of low toxicity and has no accumulation effect, but a large dose (1.5g/kg) is given to rats orally every day. ) lactic acid, causing weight loss, anemia, and increased carbon dioxide levels in the blood. Concentrated lactic acid solution can burn the skin and cause turbidity and gangrene in the cornea. Pay attention to protecting the skin and eyes when using it.
Ecological data
NoneA mixture of �� and ammonium bisulfate. The mixture enters the esterification reactor and is esterified with methanol. After ammonium bisulfate is separated, the crude ester is sent to the distillation tower, and the refined ester is obtained at the bottom of the tower. The refined ester is then sent to the distillation tower and decomposed by heating to obtain dilute lactic acid. Finally, the product is obtained through vacuum concentration.
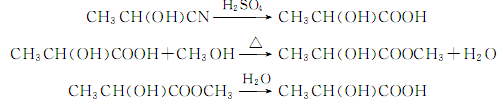
4. Tobacco: BU, 26 . In the food industry, it is prepared by the fermentation of glucose, starch, and milk; acetaldehyde reacts with hydrocyanic acid to generate cyanoethanol, which is then hydrolyzed to produce crude lactic acid. Acetolactic acid is converted into ethyl lactate, and then hydrolyzed into pure lactic acid.
Purpose
1. It can be used as a sour agent with preservative effect in the food industry. It can be used in alkyd resins in the chemical industry to help improve quality. It can also be used to prepare food emulsifiers. In the printing and dyeing industry, it can be used as a preventive agent for phenylamine to prepare printing dye slurry, etc. In the leather industry, it can be used for leather deashing, which can shorten tanning time and improve leather quality. It can also be used to produce biodegradable polymer materials such as polylactic acid. Used as a solvent for non-water-soluble dyes such as phenazine blue and alcohol-soluble sky blue. In the food industry, it is used as a general sour agent, bactericide, preservative, antifungal agent, etc. Used as mordant and solvent in dyeing industry. Used as deliming agent in leather industry.
2.Mainly used as a conditioner and emollient for skin or hair, and an acidifier to adjust the pH value. It is used in skin care creams and lotions, in hair care products such as shampoos and conditioners, and in shaving products and detergents.
3.Food sour agent, preservative, preparation of lactate, plasticizer and used in tanning, wool spinning, Printing and dyeing, pharmaceutical and other industries. Used in cosmetics to make acidic creams.
4.Lactic acid is used as a complexing agent for chemical nickel and other metals, and can also be used as an additive for electroplating.
5. Originally obtained from kefir, hence the name lactic acid. Lactic acid is widely distributed in nature. Lactic acid is produced in fermented feed and pickled sauerkraut.
6. Lactic acid is used as a calcium remover in industry, mainly in fruity and dairy flavors. Used in cheese, meat products, and dairy products.

 微信扫一扫打赏
微信扫一扫打赏

