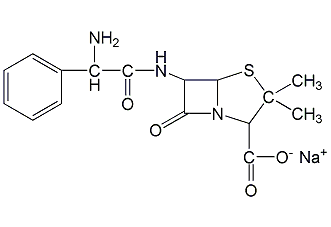
Structural formula
| Business number | 01FN |
|---|---|
| Molecular formula | C16H18N3NaO4S |
| Molecular weight | 371.38 |
| label |
ampicillin sodium, (2S,5R,6R)-3,3-dimethyl-6-[(R)-2-amino-2-phenylacetamido]-7-oxo-4-thia-1-azabicyclo[ 3.2.0]Heptane-2-carboxylic acid sodium salt, Sodium [2S-[2α,5α,6β(S*)]]-6-(aminophenylacetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate , D-(-)-α-Aminobenzylpenicillin sodium salt |
Numbering system
CAS number:69-52-3
MDL number:MFCD00064313
EINECS number:200-708-1
RTECS number:XH8400000
BRN number:4119211
PubChem number:24891463
Physical property data
1. Characteristics: White or off-white powder or crystal. Odorless or slightly odorless, slightly bitter in taste.
2. Density ( g/mL,25/4℃): Undetermined
3. Relative vapor density (g/mL,Air=1): Undetermined
4. Melting point ( ºC): 205(decomposition)
5. Boiling point ( ºC,Normal pressure): Undetermined
6. Boiling point ( ºC,5.2kPa): Undetermined
7. Refractive Index: Undetermined
8. Flashpoint (ºC): Undetermined
9. Specific optical rotation (º,C=0.2, in the water):+209°
10. Autoignition point or ignition temperature (ºC): Not OK
11. Vapor pressure (kPa,25ºC116
6. Heavy atoms Quantity: 25
7. Surface charge :0
8. Complexity :568
9. Isotope atomic number:0
10. Determine the number of atomic stereocenters:4
11. Uncertain number of atomic stereocenters:0
12. Determine the number of stereocenters of chemical bonds:0
13. Uncertain number of chemical bond stereocenters:0
14. Number of covalent bond units: 2
Properties and stability
None
Storage method
This product should be sealed in4℃Preserve dry. Validity period2year and a half.
Synthesis method
Obtained from the salt formation of ampicillin: suspend ampicillin in water and adjust with sodium hydroxide solutionpHto9, dissolve, filter. Add activated carbon to the filtrate to decolorize, filter, and dry the filtrate at low temperature to obtain the product.
Purpose
For molecular biology and tissue culture (to prevent microbial contamination). Ampicillin sodium is the bactericidal ingredient in this product. It acts on the active reproduction stage of bacteria and plays a bactericidal effect by inhibiting the biosynthesis of cell wall mucopeptides.
font-family: Arial”>Determine the number of stereocenters of chemical bonds:0
13. Uncertain number of chemical bond stereocenters:0
14. Number of covalent bond units: 2
Properties and stability
None
Storage method
This product should be sealed in4℃Preserve dry. Validity period2year and a half.
Synthesis method
Obtained from the salt formation of ampicillin: suspend ampicillin in water and adjust with sodium hydroxide solutionpHto9, dissolve, filter. Add activated carbon to the filtrate to decolorize, filter, and dry the filtrate at low temperature to obtain the product.
Purpose
For molecular biology and tissue culture (to prevent microbial contamination). Ampicillin sodium is the bactericidal ingredient in this product. It acts on the active reproduction stage of bacteria and plays a bactericidal effect by inhibiting the biosynthesis of cell wall mucopeptides.
The bactericidal ingredients in �� act on the active reproduction stage of bacteria and exert a bactericidal effect by inhibiting the biosynthesis of cell wall mucopeptides.

 微信扫一扫打赏
微信扫一扫打赏

