Background and overview[1]
The molecular formula of phloroglucinol is C6H6O3, white rhombus crystal. Sweet. Color disappears when exposed to light. Sublime and decompose at the same time. The melting point is 218°C, the relative density is 1.46, and the UVλmax269nm in ethanol. K=4.5×10((-10)). Easily soluble in alcohol, ether, benzene and pyridine, 1g dissolves in 100 parts of water. Toxicity LD504550mg/kg (oral administration to mice). Tautomerization of enols and ketones can occur, so the reaction can be carried out in either the keto form or the enol form, and substitution reactions on the benzene ring can also occur.
Phloroglucinol is a myotropic, non-atropine, non-papaaverine smooth muscle antispasmodic agent. The active ingredient is 1,3,5-trihydroxybenzene. The half-life of intravenous injection is about 15 minutes. Phloroglucinol can directly act on the smooth muscles of the gastrointestinal tract, biliary tract and urinary tract to relieve smooth muscle spasm. Its mechanism of action is not yet completely clear. In vivo and in vitro studies have shown that phloroglucinol selectively and directly relaxes the smooth muscles of some organs without causing anticholinergic adverse reactions, lowering blood pressure, increased heart rate, arrhythmia and other symptoms, and has a negative impact on cardiovascular function. There is no impact, the incidence of adverse reactions such as gastrointestinal tract and dizziness is less than 1%, and allergic reactions are rare. Toxicity test studies have found that phloroglucinol has no teratogenic or mutagenic (carcinogenic) effects.
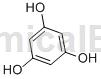
Structure
Function[2]
Compared with other smooth muscle antispasmodics, phloroglucinol’s biggest feature is that it does not have anticholinergic effects. While relieving smooth muscle spasm, it will not produce a series of anticholinergic adverse effects, nor will it cause Symptoms such as hypotension, increased heart rate, and arrhythmia have minimal impact on cardiovascular function. It only acts on spastic smooth muscle and has little effect on normal smooth muscle.
1) Effect on spasm of smooth muscle tissue. People have known about phloroglucinol for more than a century. The medical community has been conducting a large number of pharmacological studies in animals both in vivo and in vitro. Various myotropic spasmotropic agents are used to induce various smooth muscle spasms to observe the antispasmodic effect of phloroglucinol. It was only in the 1990s that the clinical efficacy of the drug in treating smooth muscle spasmodic colic was further confirmed. There are no atropine-like adverse reactions, very few other adverse reactions, and it is well tolerated.
2) Effect on physiological contraction of smooth muscle. Phloroglucinol has no effect on physiological contractions of the gastrointestinal tract, biliary tract, biliary sphincter, urethra and uterus at doses sufficient to produce an antispasmodic effect.
3) Effect on cardiovascular system. Ploroglucinol does not change the animal’s blood pressure, heart rate, and cardiac blood supply.
4) Effect on bile secretion: Low-dose phloroglucinol will not change the amount and composition of bile secretion. At higher doses, it can increase bile secretion without changing its composition.
Pharmacokinetics[3]
After intravenous injection, the half-life of the blood drug concentration is about 15 minutes. Within 4 hours after administration, the blood drug concentration decreases quickly and then decreases slowly. Fifteen minutes after administration, the concentration of the drug was highest in the liver, kidneys and small intestine, and extremely low in the brain tissue. Only a small amount of drug remained after 48 hours. The drug is metabolized in the body mainly through coupling with glucose in the liver, and all drugs are excreted in the form of glucose conjugates through the urinary tract and feces.
Indications[3]
It is mainly used to treat acute spasmodic pain caused by dysfunction of the digestive system and biliary tract, acute spasmodic urethra, bladder, renal colic, and spasmodic pain in women’s reproductive system organs.
Usage and dosage[3]
Intramuscular or intravenous injection: 40 to 80 mg each time, 40 to 120 mg daily. Intravenous infusion: The daily dose can be up to 200mg, diluted with 5% or 10% glucose injection solution and then infused.
Adverse reactions[3]
Rarely, allergic reactions such as rash, urticaria, etc. may occur.
Notes[3]
Contraindicated for those allergic to this drug. Use with caution in pregnant and lactating women.
Drug interactions[3]
1. Due to physical and chemical reactions, this injection cannot be mixed with metamizole in the same injection syringe (it may cause thrombophlebitis).
2. Avoid combined use with morphine and its derivatives, as these drugs have spasmodic effects.
Other applications�[4]
In addition to its main medical value, phloroglucinol can also be used as a fuel coupling agent and can be used in the synthesis of new phthaleous fuels. Phloroglucinol can also be used as a stabilizer for various systems, such as glutaraldehyde solution, synthetic rubber, and composite modified binary fuel rocket propellant. It can also be widely used in raw materials such as tire tackifiers and azo composite inks. It is used as a fuel coupling agent in textile and leather dyeing processes, in the production of plastic capsules, as a substitute for silver iodide for artificial rainfall, and as a preservative for some synthetic materials. The most important uses are in diazo copying, textile dyeing and the synthesis of brass isoflavone anti-tumor drugs. This product has good sales prospects in the domestic market.
Preparation[4]
A preparation method of phloroglucinol, the preparation method includes the following steps:
a. Bromination reaction: Using resorcinol as raw material, first add resorcinol into the reaction vessel, then add the reaction solvent to dissolve it, and then dissolve N-bromosuccinimide in the reaction vessel The N-bromosuccinimide solution obtained in the solvent is added dropwise into the reaction vessel. The N-bromosuccinimide solution is added for 30 to 40 minutes. After the addition is completed, the temperature is raised to 40 to 80°C. React under this temperature condition for 0.5 to 3 hours. After the reaction, a reaction liquid is obtained. After processing the reaction liquid, 4-bromoresorcinol is obtained; the ratio of the added amount between the resorcinol and the reaction solvent is 1 g. Resorcinol is added to the reaction solvent in 2 to 6 mL, and the ratio between the N-bromosuccinimide and the reaction solvent is 0.8 to 1.2 mL of the reaction solvent per gram of N-bromosuccinimide. , the mass ratio between the resorcinol and N-bromosuccinimide is 1:1.6~1.8;
b. Hydrolysis reaction: Add the 4-bromoresorcinol obtained in step a into the reaction vessel, then add the reaction solvent, strong base and catalyst, raise the temperature to 100-160°C and reflux for 4-10 hours; reaction Finally, the reaction product is cooled to below 60° C., water is added, stirred and cooled to room temperature, and the catalyst is removed by filtration. The reaction liquid obtained after filtration is left to stand for layering. After layering, the water phase is taken to obtain a phloroglucinol salt solution; described in 4 -The added amount between bromoresorcinol and the reaction solvent is 1.2 to 1.6 mL of the reaction solvent per gram of 4-bromoresorcinol, and the amount added between the 4-bromoresorcinol and the strong base The mass ratio is 1:1.04~1.06, and the mass ratio between the 4-bromoresorcinol and the catalyst is 20~22:1;
c. Acidification reaction: Add the phloroglucinol salt solution obtained in step b into the reaction vessel, and use hydrochloric acid with a concentration of 0.5 to 0.7 mol/L to adjust its pH to 1 to 3 under stirring conditions. The solid is precipitated under the conditions, and after the solid precipitation is completed, it is filtered to obtain crude solid phloroglucinol;
d. Purification of crude product: Add the crude phloroglucinol obtained in step c into the reaction vessel, and add water and heat it to 90-100°C for full dissolution. The amount of water added is 3% of the mass of the crude phloroglucinol. ~5 times, after dissolution, add activated carbon to reflux and decolorize for 30 minutes. The amount of activated carbon accounts for 3~4% of the mass of crude phloroglucinol. After decolorization, perform hot filtration at 90~100°C. The resulting filtrate will naturally cool and crystallize. After the crystallization is completed, it is filtered, and the obtained product is washed with purified water. After washing, it is dried. After drying, phloroglucinol is obtained.
Main reference materials
[1] Compound Dictionary
[2] Pharmacological effects and clinical applications of phloroglucinol
[3] Practical Drug Handbook
[4] CN201310622931.0 Preparation method of phloroglucinol

 微信扫一扫打赏
微信扫一扫打赏

