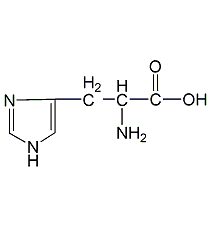
Structural formula
| Business number | 01GC |
|---|---|
| Molecular formula | C6H9N3O2 |
| Molecular weight | 155.16 |
| label |
L-A-Amino-β-4-imidazolylpropionic acid, (S)-2-Amino-3-(4-imidazolyl)propionic acid, histidine, Alpha-amino-1H-imidazole-4-propionic acid, Imidazole-5-alanine, 2-Amine-3-imidazolepropionic acid, L-2-amino-3-(4-imidazolyl)propionic acid, L-alpha-Amino-beta-imidazolepropionic acid, (S)-2-Amino-1H-imidazole-4-propanoic acid Glyoxaline-5-alanine, Flavor enhancers and nutritional supplements, amino acids, intermediates, Biochemical reagents |
Numbering system
CAS number:71-00-1
MDL number:MFCD00064315
EINECS number:200-745-3
RTECS number:MS3070000
BRN number:84088
PubChem ID:None
Physical property data
1. Properties: white crystal or crystalline powder. Odorless. Slightly bitter
2. Density (g/mL, 25/4℃): 1.423
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 287(dec.)(lit.)
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index: 13° (C=11, 6mol/L HCl)
8. Flash point (ºC): Uncertain Confirm
9. Specific rotation (º): 12.4 º (c=11,6N HCl)
10. Autoignition point or ignition temperature (ºC): Uncertain
p>
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ /mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Oil and water Log value of (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V /V): Uncertain
19. Solubility: soluble in water (4.3g/100ml, 25℃), extremely insoluble in ethanol, insoluble in ether
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 38.01
2. Molar volume (cm3/mol): 108.9
3. Isotonic specific volume (90.2K ): 325.6
4. Surface tension (dyne/cm): 79.6
5. Polarizability (10-24cm3): 15.07
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -3.2
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 4
p>
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 2
6. Topological molecular polar surface area (TPSA): 92
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 151
10. Isotopic atoms Quantity: 0
11. Determine the number of atomic stereocenters: 1
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond establishment Number of stereocenters: 0
14, Number of uncertain chemical bond stereocenters: 0
15, Number of covalent bond units: 1
Properties and stability
1. Found in flue-cured tobacco leaves and burley tobacco leaves.
Storage method
①The basic amino acid part of protein hydrolyzate is separated by ion exchange resin.
② It is made from sugar as raw material and produced by fermentation method.
Fermentation of induced drug-resistant strains of Glucobrevibacterium glutamicum, Corynebacterium glutamicum, S. marcescens, etc.
L-Histidine
Synthesis method
1. Extracted from pig blood and cow blood. Pig blood is spray-dried to produce blood meal. Every 100kg of pig blood is about 18kg of blood meal. L-Histidine is commonly used as its hydrochloride ([7048-02-4]). Concentrate the eluate containing L-histidine until crystallization appears, adjust the pH to 2.5 with hydrochloric acid while hot, immediately add ethanol twice the amount of the solution, let it stand, precipitate, and filter to obtain L-histidine. The crude hydrochloride salt is decolorized, recrystallized and dried to obtain the finished product. L-histidine can also be extracted from the hydrolyzate of defatted soybeans.
2.Direct fermentation method

3. Tobacco: BU, 22; FC, 21. Its imidazole easily forms complex salts with metal ions.
Purpose
1. Mainly used as flavor enhancer and nutritional supplement. It can also be used in food for infants and young children and food for post-operative patients. It is also an important component of amino acid infusion and comprehensive amino acid preparations.

 微信扫一扫打赏
微信扫一扫打赏

