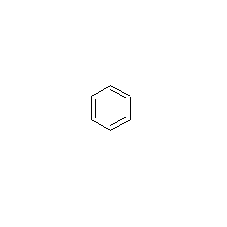
Structural formula
| Business number | 01GH |
|---|---|
| Molecular formula | C6H6 |
| Molecular weight | 78.11 |
| label |
Benzol, Cyclohexatriene, Dehydrating solvent for organic synthesis, thinner, paint stripper, Aromatic hydrocarbons |
Numbering system
CAS number:71-43-2
MDL number:MFCD00003009
EINECS number:200-753-7
RTECS number:CY1400000
BRN number:969212
PubChem number:24856389
Physical property data
1. Properties: colorless and transparent liquid with strong aromatic smell. [1]
2. Melting point (℃): 5.5[2]
3. Boiling point (℃): 80.1 [3]
4. Relative density (water = 1): 0.88[4]
5. Relative vapor density (Air=1): 2.77[5]
6. Saturated vapor pressure (kPa): 9.95 (20℃)[6]
7. Heat of combustion (kJ/mol): -3264.4[7]
8. Critical temperature (℃): 289.5[8]
9. Critical pressure (MPa): 4.92[9]
10. Octanol/water partition coefficient: 2.15[ 10]
11. Flash point (℃): -11[11]
12. Ignition temperature (℃): 560 [12]
13. Explosion upper limit (%): 8.0[13]
14. Explosion lower limit (%) : 1.2[14]
15. Solubility: Insoluble in water, soluble in most organic solvents such as ethanol, ether, acetone and so on. [15]
16. Refractive index (25ºC): 1.49794
17. Viscosity (mPa·s, 25ºC): 0.6010
18. Heat of evaporation (KJ/mol, 25ºC): 33.9
19. Heat of fusion (KJ/mol): 9.872
20. Heat of formation (KJ/mol, 25ºC) , gas): 82.966
21. Heat of formation (KJ/mol, 25ºC, liquid): 49.051
22. Heat of combustion (KJ/mol, 25ºC, gas): 3303.08
23. Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure): 1.05
24. Boiling point elevation constant: 2.53
25. Electrical conductivity Rate (S/m): 76×10-9
26. Thermal conductivity (W/(m·K), 25ºC): 0.1442
27. Vapor pressure (kPa, 79.5ºC): 41.3
28. Volume expansion coefficient (K-1): 0.00121
29. Critical density (g·cm-3): 0.305
30. Critical volume (cm3·mol-1): 256
31. Critical compression factor: 0.268
32. Eccentricity factor: 0.211
33. Lennard-Jones parameter (A): 5.3823
34.Lennard-Jones parameter (K): 426.70
35. Solubility parameter (J·cm-3)0.5: 18.706
36. van der Waals area (cm2·mol-1): 6.000×109
37.van der Waals volume (cm3·mol-1): 48.400
38. Gas phase standard heat of combustion (enthalpy) (kJ·mol-1): -3301.47
39. The gas phase standard claims heat (enthalpy) (kJ·mol-1): 82.89
40. Gas phase standard entropy (J·mol-1·K-1): 269.30
41. Gas phase standard generation Free energy (kJ·mol-1): 129.8
42. Gas phase��The raw materials are mixed with circulating hydrogen and then enter the heating furnace. The heated mixed gas enters the fixed-bed catalytic reactor installed in series. After the reaction product is cooled by heat exchange, it enters the high-pressure gas-liquid separator. The gas product passes through the circulating compressor After compression, it is recycled. The liquid is extracted with diethylene glycol ether, an industrial solvent, to separate aromatic hydrocarbons from non-aromatic hydrocarbons, and then sent to the distillation tower to collect the 79.7-80.5°C fraction, which is pure benzene.
(3). Pyrolysis gasoline to benzene produced by pyrolysis gasoline generally contains 40% to 70% aromatic hydrocarbons. Aromatic hydrocarbons contain about 37% benzene, about 14% toluene, and about 5% xylene. Benzene is extracted by hydrodealkylation. First, pyrolysis gasoline is subjected to two-stage catalytic hydrogenation, catalytic dealkylation and hydrogen purification to convert alkylbenzene into benzene, and then benzene is obtained through fractionation.
Continuously wash the industrial benzene with concentrated sulfuric acid to remove the impurity thiophene content, then wash it with water and 5% sodium hydroxide solution, dry it with anhydrous calcium chloride, and distill the supernatant liquid.
(2) According to the sulfide content in industrial benzene, add an appropriate amount of 42% sodium hydroxide solution, 95% ethanol and lead acetate, and mix well. Continue stirring until the sulfide test is qualified (measure the sulfide content every 2.5 hours). Stop stirring and let it sit for 1 hour. After releasing the lower sodium hydroxide solution, wash it with sufficient distilled water 3 to 5 times, stirring for 0.5 hours each time, and let it stand. Leave for 0.5h to separate the lower water layer. Then add an appropriate amount of concentrated sulfuric acid under stirring, wash the benzene liquid until the thiophene content is qualified, stir for 2 hours each time, let it stand for 0.5 hours, and then release the lower acid liquid. After pickling, wash twice with water, then add 20% sodium hydroxide solution, stir for 0.5h, let it stand, and separate the alkali layer until it is qualified. Then dry and dehydrate using calcium chloride. The dehydrated benzene is distilled, and the clear distillate is collected as the finished product.
(3) Use coking by-product recovery method, platinum reforming separation method and pyrolysis gasoline extraction method. The coking by-product recovery method uses crude benzene recovered from coking by-products as raw material, which is obtained by initial distillation and then rectification. The platinum reforming separation method is to use the light gasoline obtained by atmospheric distillation to first undergo catalytic hydrogenation, then reform with platinum, extract with diethylene glycol ether, and finally be separated by distillation. The pyrolysis gasoline extraction method uses pyrolysis gasoline as raw material, converts alkylbenzene into benzene by hydrodealkylation method, and then obtains it through fractionation.
Purpose
1. Used as an important raw material for synthetic dyes, synthetic rubber, synthetic resin, synthetic fibers, synthetic grains, plastics, medicines, pesticides, photographic films and petrochemical products. This product has good solubility and is therefore widely used Used as adhesives and industrial solvents such as varnish, nitrocellulose paint thinner, paint stripper, lubricating oil, grease, wax, celluloid, resin, artificial leather and other solvents.
2. Standard sample for measuring refractive index. It can be used as solvent and cleaning agent for precision optical instruments, electronic industry, etc., organic synthesis, etc.
3. Used as analytical reagents, such as solvents and standard materials for chromatographic analysis.
4. Cosmetic solvents. It is mainly used as a diluent for cosmetics such as nail polish to accelerate drying and hardening, and to increase the solubility of skin film components such as resin.
5. Used as solvents and synthetic benzene derivatives, spices, dyes, plastics, medicines, explosives, rubber, etc. [31]

 微信扫一扫打赏
微信扫一扫打赏

