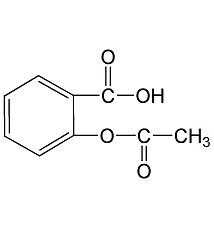
Structural formula
| Business number | 0145 |
|---|---|
| Molecular formula | C9H8O4 |
| Molecular weight | 180 |
| label |
acetyl salicylic acid, aspirin, Acesalicylic acid, Acetoxybenzoic acid, Acenterine, Acetophen, 2-(Acetoxy)benzoic acid, O-Acetylsalicylic acie, Aspro |
Numbering system
CAS number:50-78-2
MDL number:MFCD00002430
EINECS number:200-064-1
RTECS number:VO0700000
BRN number:779271
PubChem number:24278218
Physical property data
1. Properties: White needle-like or flaky crystals. Odorless. The slightly sour smell is stable in dry air and gradually hydrolyzes into salicylic acid and acetic acid in humid air.
2. Density (g/mL, 25/4℃): 1.1872154
3. Relative density (20℃, 4℃): 1.40
4. Melting point (ºC): 135
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa) : Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12 . Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
p>
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in ethanol, ether, slightly Dissolved in water.
Toxicological data
Acute toxicity:
Oral LD50 700mg/kg(dog)
1075mg/kg(guinea pig)
3500mg/kg(ham)
p>
1750mg/kg(mam)
250mg/kg(mus)
200mg/kg(rat)
1010mg/kg(rbt)
LDLo 104mg/kg(chd)
p>
Main irritant effects:
On skin: Irritation to skin and mucous membranes.
On eyes: Irritation effects
Sensitization: No known sensitizing effects.
Ecological data
General remarks
Water hazard level 1 (German regulations) (self-assessment via list) This substance is slightly hazardous to water.
Do not allow undiluted or large quantities of product to come into contact with groundwater, waterways or sewage systems.
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 44.52
2. Molar volume (cm3/mol): 139.5
3. Isotonic specific volume (90.2K ): 370.9
4. Surface tension (dyne/cm): 49.8
5. Polarizability (10-24cm3): 17.65
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 1.2
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 3
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 63.6
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 212
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Toxic if taken orally. Appropriate protective clothing should be worn if used in large quantities.
Storage method
1. Production equipment should be sealed and good ventilation should be maintained in the workshop.
2. Store away from light and sealed in a dry place.
Synthesis method
1. Obtained by acetylation of salicylic acid: Add acetic anhydride to the reaction tank (the amount added is 0.7889 times the total amount of salicylic acid), then add two-thirds of the amount of salicylic acid, stir and raise the temperature, React at 81-82°C for 40-60 minutes. Lower the temperature to 81-82°C and keep the reaction for 2 hours. After checking that the free salicylic acid is qualified, the temperature is lowered to 13°C, crystals are precipitated, filtered, washed and dried, and air-dried at 65-70°C to obtain acetylsalicylic acid.
2. Preparation method:
In a dry reaction bottle, add 25g (0.18mol) of dry salicylic acid (2), 38g (0.37mol) of acetic anhydride, concentrated Add 1 mL of sulfuric acid and react in a 60°C water bath for 30 minutes. After cooling, pour into 400 mL of water, stir thoroughly, filter and wash with water. Recrystallize with 1:1 dilute acetic acid ① to obtain 32g of acetylsalicylic acid (1), with a yield of 98%. Note: ① The following method can also be used for purification: Dissolve the crude product in a small amount of hot ethanol, then slowly add the hot ethanol solution to 2.5 times the volume of hot water to form a transparent solution, and slowly cool it to precipitate needle-like crystals of acetylsalicylic acid. . [1]
3. Preparation method:
Add 25g (0.18mol) of dry salicylic acid (2) into the reaction bottle. 18 mL of pyridine, shake to dissolve. Slowly add 21g (0.28mol) of acetyl chloride dropwise, the reaction will be exothermic, and the reaction temperature should not exceed 60°C. After the addition, continue the reaction for 10 minutes. Cool, pour into 60mL cold water, and solid will precipitate. Filter, wash with water, and then recrystallize with 1:1 dilute acetic acid to obtain 22 g of acetylsalicylic acid (1), with a yield of 67.5%. mp136~138℃ (partial decomposition at 128~135℃). [2]
Purpose
1. Acetylsalicylic acid is the raw material for preparing the rodenticide intermediate 4-hydroxycoumarin.
2. Used to manufacture structural parts and equipment components outdoors and exposed to strong light, such as automobile bodies, agricultural machinery parts, electricity meters and lampshades, road markings, etc.
3. Solution Thermal analgesics are used for fever, pain and rheumatoid arthritis. They are the earliest, most widely used and most common antipyretic, analgesic and anti-rheumatic drugs. It has many pharmacological effects such as antipyretic, analgesic, anti-inflammatory, anti-wind temperature and anti-platelet aggregation. It exerts rapid effects and is sure to be effective. Excessive dosage is easy to diagnose and handle, and allergic reactions rarely occur. Commonly used for colds, fever, headache, neuralgia, joint pain, myalgia, rheumatic fever, acute endometritis, rheumatoid arthritis and toothache. Acetylsalicylic acid is a variety listed in the “National Essential Drugs List” and is also an intermediate for other drugs.
4. Pharmaceutical industry, organic synthesis, reagent for detecting manganese.

 微信扫一扫打赏
微信扫一扫打赏

