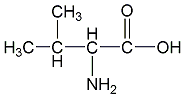
structural formula
| business number | 01gw |
|---|---|
| molecular formula | c5h11no2 |
| molecular weight | 117.15 |
| label |
2-amino-3-methylbutyric acid, l-andrographis amino, l-isovalerine, alpha-amine isovaleric acid, 2-aminoisovaleric acid, a-aminoisovaleric acid, 2-aminoisovaleric acid, -2-amino-3-methylbutyric acid, l-(+)-.alpha.-aminoisovaleric acid, 2-amino-3-methylbutanoic acid, amino acid drugs, intermediates, biochemical reagents |
numbering system
cas number:72-18-4
mdl number:mfcd00064220
einecs number:200-773-6
rtecs number:yv9361000
brn number:1721136
pubchem number:24900695
physical property data
1. properties: white crystal or crystalline powder, odorless, bitter
2. density (g/ml, 25/4℃): 1.23
3. relative vapor density (g/ml, air=1): uncertain
4. melting point (ºc): 295-300 (subl.)(lit.)
5. boiling point (ºc, normal pressure): uncertain
6. boiling point (ºc, 5.2 kpa): uncertain
7. refractive index: 28 ° (c=8, hcl)
8. flash point (ºc): uncertain
9. specific rotation (º): 28 º (c=8, 6n hcl)
10 . autoignition point or ignition temperature (ºc): uncertain
11. vapor pressure (kpa, 25 ºc): uncertain
12. saturated vapor pressure (kpa, 60 ºc ): uncertain
13. heat of combustion (kj/mol): uncertain
14. critical temperature (ºc): uncertain
15. critical pressure (kpa): uncertain
16. log value of oil-water (octanol/water) distribution coefficient: uncertain
17. explosion upper limit (%, v/v): uncertain
18. lower explosion limit (%, v/v): uncertain
19. solubility: easily soluble in water, solubility (g/l): 0℃ 83.4 ; 25℃ 88.5; 50℃ 96.2; 65℃ 102.4. slightly soluble in ethanol, insoluble in ether.
toxicological data
acute toxicity: rat abdominal ld50: 5390 mg/kg; mutagenicity: microbial mutation test systems – not otherwise specifiedtest system: 10mmol/l
ecological data
none
molecular structure data
1. molar refractive index: 30.23
2. molar volume (cm3/mol): 110.1
3. isotonic specific volume (90.2k ): 276.6
4. surface tension (dyne/cm): 39.8
5. polarizability (10-24cm3): 11.98
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 2
3. number of hydrogen bond acceptors: 3
4. number of rotatable chemical bonds: 2
5. number of tautomers: none
6. topological molecule polar surface area 63.3
7. number of heavy atoms: 8
8. surface charge: 0
9. complexity: 90.4
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 1
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
1. exists in flue-cured tobacco leaves, burley tobacco leaves and smoke.
storage method
this product should be kept sealed, cool, dry and away from light.
synthesis method
1. it can be obtained by condensation of 2-aminothiazole and p-nitrobenzenesulfonyl chloride and then reduction.
2. using isobutyraldehyde as raw material, there are many methods to synthesize racemic valine. for example, isobutyraldehyde reacts with ammonia to form aminoisobutanol, and then reacts with hydrogen cyanide to form aminoisobutyronitrile, which is then hydrolyzed to obtain valine. there are also many ways to resolve the racemic form, such as using acyl-dl-amino acids. the enzyme hydrolyzes the amino acid, and then uses the solubility difference between the free amino acid and the acylated form to separate. the valine produced by fermentation method is all l-form and does not require optical resolution. the bacterial species used in the fermentation method are micrococcus glutamicum, brevibacterium ammoniagenes, escherichia coli, and aerobacter aerogenes. use culture media such as glucose, urea, and inorganic salts.
3.fermentation method

4. tobacco: bu, 22; fc, 21; synthesis: it can be obtained by hydrolysis and refinement of fish protein, etc., or it can also be synthesized by chemical methods .
purpose
1. it is an essential amino acid. the required amount for adult men is 10 mg/(kg·d). the physiological effect of l-type is twice that of d-type. lack of it can cause neurological disorders, cessation of growth, weight loss, anemia, etc. as a nutritional supplement, it can be used together with other essential amino acids to prepare amino acid infusions and comprehensive amino acid preparations. valine (1g/kg) is added to rice cakes, and the product has a sesame aroma. it can also improve the flavor when used in bread.
2.amino acid drugs. nutritional supplement, can be used as the main component of amino acid infusion and comprehensive amino acid preparations.

 微信扫一扫打赏
微信扫一扫打赏

