Background and overview[1-2]
Yellow to light brown oily liquid. Slightly pungent odor. Relative molecular mass 121.18. Relative density 0.9557. Melting point 2.45℃. Boiling point: 194.5℃, 153.4℃ (33.331×103Pa), 100℃ (4.533×103Pa), 77℃ (1.733×103 sup>Pa). Flash point 62℃. Refractive index 1.5582. Insoluble in water, soluble in ethanol, ether, acetone, chloroform and aromatic hydrocarbon solvents, soluble in acid. Inhalation of vapor produces symptoms of poisoning. Rat oral LD501410mg/kg. The usual manufacturing method of dimethylaniline is to mix aniline, methanol and sulfuric acid in a certain proportion and react in an autoclave at 200-230°C and 3.0-4.0MPa to generate dimethylaniline. Since the reaction is reversible process, and passes through the intermediate N-methylaniline (hereinafter referred to as MA). Affected by the equilibrium constant, the crude dimethylaniline usually contains a certain amount of unreacted raw material aniline (hereinafter referred to as An) and the intermediate MA, China The Ministry of Chemical Industry standard HG2-375-83 stipulates that the MA content in first-grade dimethylaniline must be ≤0.5% (w/w, the same below), and the An content must comply with the bleaching powder test standard (HG2-375-83 Article 2.4 , equivalent to ≤0.03%). Japanese standard JISK4112 and Taiwanese standard CNS12196-K2180 also have the same regulations on the MA and An content in finished dimethylaniline products as the ministry standard HG2-375-83.
Purpose[3-5]
Dimethylaniline is used as a raw material for organic dyes to produce basic dyes, disperse dyes, acid dyes, and oil-soluble dyes. These dyes have not been used due to their poor fastness when dyeing clothes, but due to their hue characteristics It is used for dyeing and ink of paper, pulp, leather, groceries, etc. However, it is widely used as a functional pigment, such as blue pressure-sensitive pigment and melanin for carbon paper. In addition, it is also used as solvents, pharmaceutical raw materials; raw materials for ultraviolet absorbers, photosensitizers, reagents, Micher and fragrances (vanillin) in cosmetics. Examples of its application are as follows:
1) The N, N-dimethylaniline method is used to continuously produce trimethyl phosphite, which belongs to the technical field of trimethyl phosphite production. Through the steps of synthesis, water washing, layering, drying, distillation, waste water recovery, etc., the present invention has low sewage treatment costs and ensures the product quality and production volume of trimethyl phosphite. After the sewage is concentrated and crystallized, it can be widely used for chlorination. Ammonium fertilizer. Overcome the problems of high energy consumption, large amount of alkali used, high production cost, and wastewater containing a large amount of sodium chloride in the continuous production of trimethyl phosphite from triethylamine. The concentrated sodium chloride cannot be used. It can also solve the shortcomings of the batch method, which is only suitable for small-scale enterprise production, with small output, low yield and large environmental pollution. In addition, the present invention separates the product from the solvent in the water washing liquid through a centrifugal separator, and effectively removes the amine chloride salt through the second alkali washing, which effectively solves the problem of the amine chloride salt clogging the distillation equipment.
2) Preparation of methylene blue belongs to the field of fine chemical intermediate synthesis technology. The specific steps are to use sodium nitrite and N,N-dimethylaniline to carry out nitrosation reaction in concentrated hydrochloric acid solution to prepare the intermediate p-nitroso-N,N-dimethylaniline; p-nitroso -N,N-dimethylaniline is hydrogenated and reduced to prepare p-amino-N,N-dimethylaniline; p-amino-N,N-dimethylaniline is oxidized and then added with sodium thiosulfate. into 2-amino-5-dimethylaminophenyl thiosulfonic acid; 2-amino-5-dimethylaminophenyl thiosulfonic acid is oxidatively condensed with N, N-dimethylaniline to form bis( 4-Dimethylaminophenyl)thiosulfonic acid; perform a ring-closure reaction on bis(4-dimethylaminophenyl)thiosulfonic acid to obtain methylene blue. The preparation method provided by the invention has high product purity, simple process flow, low manufacturing cost, is suitable for industrial production, the raw materials used are easy to obtain, and causes less environmental pollution.
3) Synthesis of N-arylcarboxamide compounds, the steps are: N, N-dimethylaniline compound, cuprous chloride, sodium tetrafluoroborate and salicylic acid are placed in an organic Among the solvents, the amount of the organic solvent used is: N, N-dimethylaniline compound, cuprous chloride, sodium tetrafluoroborate and salicylic acid, the substance concentration is 0.5~1mol/L, 30~ The reaction is carried out at 60°C for 0.5 to 48 hours, the product is separated and purified, and N-arylcarboxamide compounds are obtained; the present invention has the advantages of simple operation, cheap catalysts, mild reaction conditions, and high product yield, and overcomes the traditional problems of expensive raw material reagents, It has the advantages of harsh conditions, lengthy synthesis steps, and low overall yield, but has good application prospects.
Preparation[6]
1. Liquid phase synthesis method
The traditional preparation method is to use sulfuric acid as a catalyst and react aniline and methanol in a high-pressure reactor at high temperature and high pressure. The main disadvantage of this method is that the reaction under high pressure requires the selection of corrosion-resistant and high-pressure resistant equipment materials, which increases investment. The separation product requires the use of a large amount of liquid alkali to neutralize the inorganic acid, and produces a large amount of inorganic salts that are difficult to handle. The reaction formula is:
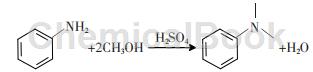
The liquid-phase synthesis of N,N-dimethylaniline requires a higher activity N-methylating reagent or a higher activity catalysis. Haloalkanes also have good reaction effects in alkylation reactions, but they are highly toxic. For N-methylation reactions, methyl chloride is a gas at room temperature and is inconvenient to use. Although methyl bromide and methyl iodide are It is liquid at room temperature, but has a higher price.��
2. Gas phase synthesis method
N,N-dimethylaniline is synthesized in gas phase using aniline and methanol over beta zeolite catalyst. When n (aniline): n (methanol) = 1:3, the reaction temperature is 240~250°C, and the feed space velocity is 0.5h-1, the single-pass conversion rate of aniline is 99.6%, N, N-dimethyl The selectivity of aniline can reach 91.7%, and the catalyst life can be maintained for 800 hours. But the low airspeed becomes a shortcoming of this reaction. Suib et al. used RHO-FAU co-precipitated molecular sieve as a catalyst and aniline and methanol as raw materials to synthesize N, N-dimethylaniline by gas phase method [19]. When the molar flow rate of aniline and methanol is 1:3.5 and the reaction temperature is 400°C, the conversion rate of aniline is about 95% and the product selectivity is greater than 90%. Research shows that FAU type molecular sieve has good adsorption effect on aniline, while RHO type molecular sieve can effectively disperse FAU type molecular sieve and activate methanol. The synergistic effect of the two components makes the RHO-FAU molecular sieve show good catalytic effect in the reaction of catalyzing the gas phase synthesis of N, N-dimethylaniline.
Main reference materials
[1] Practical Fine Chemical Dictionary
[2] CN99114288.8 Method for purifying N, N-dimethylaniline using acid anhydride
[3] CN201210346113.8N, process for continuous production of trimethyl phosphite by N-dimethylaniline method
[4] CN201510465840.X Preparation method of methylene blue
[5] CN201810468279.4 A method for synthesizing N-arylcarboxamide compounds
[6] Research progress on the synthesis of N-methylaniline and N, N-dimethylaniline

 微信扫一扫打赏
微信扫一扫打赏

