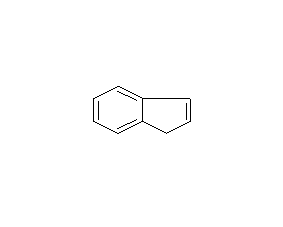
Structural formula
| Business number | 028M |
|---|---|
| Molecular formula | C9H8 |
| Molecular weight | 116.16 |
| label |
1H-indene, benzocyclopropene, 1H-Indene, Indonaphthene, Technical indene, paint solvents, Indene resin synthesis |
Numbering system
CAS number:95-13-6
MDL number:MFCD00003777
EINECS number:202-393-6
RTECS number:NK8225000
BRN number:635873
PubChem number:24851616
Physical property data
1. Properties: colorless and transparent oily liquid
2. Density (g/mL, 20/4℃): 0.9960
3. Relative density (g/mL, 50/4℃): 0.9692
4. Melting point (ºC): -2
5. Boiling point (ºC, normal pressure): 182.44
6. Refractive index (18.5ºC): 1.5773
7. Refractive index (n20ºC): 1.5764
8. Flash point (ºC): 58
9. Dissolution Properties: Insoluble in water, soluble in ethanol, ether, acetone, benzene, pyridine and other organic solvents.
10. Relative density (25℃, 4℃): 0.9949
11. Refractive index at room temperature (n25): 1.5737
12. Solubility parameter (J·cm-3)0.5: 20.467
13. van der Waals area (cm2 ·mol-1): 7.930×109
14. van der Waals volume (cm3·mol-1): 68.890
15. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -4848.3
16. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): 163.4
17. Gas phase standard entropy (J·mol– 1·K-1): 334.04
18. Gas phase standard free energy of formation (kJ·mol-1): 235.1
19. Gas phase standard hot melt (J·mol-1·K-1): 124.31
20. Liquid phase Standard heat of combustion (enthalpy) (kJ·mol-1): -4795.5
21. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): 110.6
22. Liquid phase standard entropy (J·mol-1·K-1): 214.18
23. Liquid phase standard free energy of formation (kJ·mol-1): 217.8
24. Liquid phase standard hot melt (J·mol-1 ·K-1): 186.94
Toxicological data
1. Acute toxicity: rat inhalation LC50: 14mg/m3; rat LD50: 2300mg/kg; mouse LD50: 1800mg/kg; mammal oral LD50: >5mg /kg;
2. Multiple dose toxicity: rats inhaled TCLo: 3mg/ m3/24H/15W-C; 3. It is of low toxicity. It has an irritating effect on skin and mucous membranes. High-concentration exposure can cause liver,Damage to spleen, kidneys, lungs and other organs. The olfactory threshold concentration is 0.001 mg/L, and the maximum allowable concentration in the workplace is 45 mg/m3 (USA).
Ecological data
It is not harmful to water.
Molecular structure data
1. Molar refractive index: 38.03
2. Molar volume (cm3/mol): 111.8
3. Isotonic specific volume (90.2K ): 284.8
4. Surface tension (dyne/cm): 42.1
5. Dielectric constant: 2.77
6. Dipole moment (10- 24cm3): no use
7. Polarizability: 15.07
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 124
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. This product is flammable and may burn when exposed to high temperatures. It is easily oxidized in the air and can form polymers when exposed to air and sunlight. Avoid contact with oxides and light.
2.Rat can tolerate six repeated inhalations of 3.8~4.2 mg/L of this product without visible symptoms of poisoning. The maximum allowable concentration in the air in the workplace is 240mg/m3.
3. Exist in smoke.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. Keep away from fire sources and prevent static electricity. The packaging is sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills. 2. This product is flammable. There is a risk of burning when exposed to open flames or high heat. It should be stored in a cool, well-ventilated warehouse away from heat sources and fires. It should be stored separately from oxidants. Pack and unload with care, and keep the packaging intact. It should not be stored for a long time to prevent deterioration.
3. Can polymerize and oxidize during storage.
Synthesis method
In high-temperature tar, the indene content is 0.25%-0.3%. It mainly exists in coal tar and crude benzene fractions with boiling points of 168-175°C. In heavy benzene before 200°C, coumaron and indene account for about More than 40%. Heavy benzene is used as raw material for rectification on a fractionation tower with 44 theoretical plates. The reflux ratio is controlled to 15-20:1, and a fraction with a tower top temperature of 180.6-181.1°C is cut, and the indene content can reach 98%. Another method of extracting indene is to use heavy benzene and crude solvent oil as raw materials, and use furfural as an azeotropic agent for azeotropic distillation. Since furfural or formaldehyde can form an azeotrope with the hydrocarbon components in heavy benzene, but does not form an azeotrope with indene, pure products can be produced.
Use heavy benzene as raw material for rectification on a fractionation tower with 44 theoretical plates. Control the reflux ratio to 15-20:1, and cut the fraction with a top temperature of 180.6-181.1°C, containing indene. Up to 98%.
Tetralin can also be produced by passing SiO2/A12O3 at high temperatures.
Purpose
Except for a small amount of intermediates used in organic solvents and pesticides, it is mainly used in the production of indene-coumarone resin. It can be mixed with other liquid hydrocarbons as coating solvents.

 微信扫一扫打赏
微信扫一扫打赏

