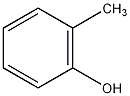
Structural formula
| Business number | 0295 |
|---|---|
| Molecular formula | C7H8O |
| Molecular weight | 108.14 |
| label |
o-cresol, O-steamed lignooleic acid, 2-Cresol, 2-methylphenol, 2-Cresol, 2-Methylphenol, o-Methylphenol, o-Hydroxy-toluene, Aromatic halogen derivatives |
Numbering system
CAS number:95-48-7
MDL number:MFCD00002226
EINECS number:202-423-8
RTECS number:GO6300000
BRN number:506917
PubChem number:24867813
Physical property data
1. Characteristics: white crystal with aromatic odor. [1]
2. Melting point (℃): 29.8~31[2]
3. Boiling point (℃) :191~192[3]
4. Relative density (water=1): 1.05[4]
5 .Relative vapor density (air=1): 3.72[5]
6. Saturated vapor pressure (kPa): 0.133 (38.2℃)[6]
7. Heat of combustion (kJ/mol): -3689.8[7]
8. Critical temperature (℃): 424.5 [8]
9. Critical pressure (MPa): 5.01[9]
10. Octanol/water partition coefficient: 1.95 [10]
11. Flash point (℃): 81 (CC) [11]
12. Ignition Temperature (℃): 598[12]
13. Explosion limit (%): 7.6[13]
14 .Lower explosion limit (%): 1.4 (148℃)[14]
15. Solubility: Slightly soluble in water, soluble in ethanol, ether, chloroform, etc. [15]
16. Viscosity (mPa·s, 20ºC): 9.56
17. Heat of evaporation (KJ/mol, b.p.): 415.0
18. Specific heat capacity (KJ/(kg·K), constant pressure): 2.09
19. Electrical conductivity (S/m, 25ºC): 0.127×10-8
20. Solubility (%, 25ºC, water): 2.2
21. Refractive index at room temperature (n25): 1.5399 p>
22. Relative density (25℃, 4℃): 1.03535
23. Eccentricity factor: 0.434
24. Gas phase standard Heat of combustion (enthalpy) (kJ·mol-1): -3769.32
25. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -128.57
26. Gas phase standard entropy (J·mol-1·K-1): 352.70
27. Gas phase standard formation free energy (kJ·mol-1): -34.3
28. Gas phase standard hot melt (J·mol-1·K-1): 127.30
29. Crystal phase standard combustion heat (enthalpy) (kJ·mol-1): -3693.30
30. Crystal phase standard claim heat (enthalpy) (kJ·mol-1): -204.60
31. Crystal phase standard entropy (J· mol-1·K-1): 165.44
32. Crystal phase standard formation free energy (kJ·mol-1): -55.69
33. Crystal phase standard hot melt (J·mol-1·K-1): 154.56
Toxicological data
1. Acute toxicity: Rat oral LD50: 121mc=”http://images.basechem.org/internal/day_100917/201009171440404499.gif” alt=”” />
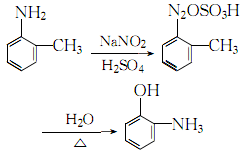
4. Add o-toluidine to the sulfuric acid solution, stir and cool to obtain o-toluidine Toluidine sulfate, add ice cubes to cool down to 0~5℃, maintain this temperature range, slowly add saturated sodium nitrite solution while stirring until the starch-potassium iodide test paper does not fade, which is the reaction end point. Add the obtained diazonium salt to concentrated sulfuric acid and stir thoroughly. Stop stirring. After the reactants react violently, add steam and heat until complete hydrolysis. Let stand and separate into layers, remove the oily layer, add 5% sodium hydroxide, filter out insoluble impurities, and then neutralize with 25% sulfuric acid until the pH value is 0. Leave to stand for layering, take the oil layer, and wash it twice with water. The crude product obtained is distilled under reduced pressure, and the 74-75.5°C fraction is collected at 1333 Pa to obtain the finished product. The process reaction formula is:
Purpose
1. Mainly used as synthetic resin. It can also be used to make pesticides, dimethyltetrachloride herbicides, pharmaceutical disinfectants, spices, chemical reagents and antioxidants. Its downstream products mainly include synthetic resin o-cresol phenolic Resin, o-methyl salicylic acid, p-chloro-o-cresol, o-hydroxybenzaldehyde, 2-methyl-5-isopropylphenol and antioxidants, etc. In addition, it can also be used as diluent, disinfectant and plasticizer in the production of sebacic acid. It can also be used as important fine chemical intermediates such as synthetic pesticides, dyes, plastic antioxidants, polymerization inhibitors and spices.
2. Used as analytical reagents and in organic synthesis. [28]

 微信扫一扫打赏
微信扫一扫打赏

