Background and overview[1]
Acid chlorides including (2-chlorophenyl)methylsulfonyl chloride are a very important class of compounds, widely used as intermediates and raw materials in organic and pharmaceutical synthesis, and are an important source of sulfonyl groups. Due to the important role and widespread use of sulfonyl chloride, a variety of preparation methods for sulfonyl chloride have been developed.
Structure
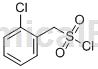
Preparation method[1]
Generally speaking, alkylsulfonyl chlorides can be prepared by the following two methods: (1) direct chlorination of alkylsulfonic acid or its salt using chlorination reagents; (2) thiols and their precursors or Derivatives (thiols, S-alkyl isothiouronium salts, disulfides, acetate thiol esters, carbamate thiol esters, etc.) are subjected to oxidative chlorination. The first method has the disadvantages of harsh reaction conditions, long reaction time, low functional group tolerance, use of excessive chlorination reagents, and the generation of acidic or toxic by-products; the second method has made great progress in the past century. progress, but still faces shortcomings. For example, some oxidative chlorination reagents are not easily available. Therefore, how to prepare sulfonyl chloride simply becomes increasingly important. There are studies on the effects of S-hydrocarbyl isothiouronium salts, thiols and thiophenols, disulfides, thiol acetate and thiophenol acetate, xanthan under acidic conditions through chlorous acid and its salts. Sulfonyl chloride is prepared through the oxidative chlorination reaction of sulfur-containing compounds such as acid esters. The process is simple to operate, has short reaction time and high yield, and the product is easy to separate. The sulfur-containing raw materials used are widely available and easy to obtain.
The preparation method of (2-chlorophenyl)methylsulfonyl chloride is as follows: Dissolve thiourea (0.387g, 5mmol) and o-chlorobenzyl chloride (5mmol) in 5mL ethanol, reflux for 30 minutes, then remove under reduced pressure The solvent gave a white solid. The white solid was slowly added dropwise to a mixed system of NaClO2 (1.61g, 15mmol, 85% purity), concentrated HCl (3mL) and MeCN (10mL). During the feeding process, use a water bath to control the temperature in the reaction system between 10 and 20oC. After the addition is completed, continue to stir the reaction for 30 minutes, remove the acetonitrile under reduced pressure at low temperature, add 25 mL of water, filter the solids in the system, and dry to obtain the product colorless crystals (2-chlorophenyl) methylsulfonyl chloride, colorless crystals , melting point 64~65℃, 1.024g, yield 91%.
1HNMR (400MHz, CDCl3) δ: 7.61~7.35 (m, 4H), 5.12 (s, 2H).
Main reference materials
[1] CN201310276839.3 A universal preparation method of sulfonyl chloride

 微信扫一扫打赏
微信扫一扫打赏

