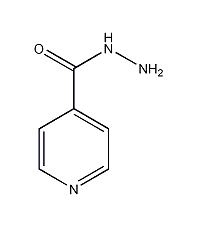
Structural formula
| Business number | 016L |
|---|---|
| Molecular formula | C6H7N3O |
| Molecular weight | 137.14 |
| label |
Isoniazid |
Numbering system
CAS number:54-85-3
MDL number:MFCD00006426
EINECS number:200-214-6
RTECS number:NS1751850
BRN number:119374
PubChem number:24896031
Physical property data
1. Character:Colorless needle-like crystals. Odorless. The taste is slightly sweet and then bitter. It gradually deteriorates when exposed to light.
2. Density (g/mL,25/4℃): Undetermined
3 . Relative vapor density (g/mL,air=1): Undetermined
4. Melting point (ºC): 171.4
5. Boiling point (ºC,Normal pressure): Undetermined
6. Boiling point (ºC,5.2kPa): Undetermined
7. Refractive index: undetermined
8. Flashpoint (ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa,25ºC): Undetermined
12.
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 4
6. Topological molecule polar surface area 68
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 120
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be sealed and stored away from light.
Synthesis method
It is obtained by the condensation of isonicotinic acid and hydrazine hydrate. Dissolve isonicotinic acid in hydrazine hydrate, add the crude mother liquor from the previous batch, and distill under reduced pressure to 79-82℃(13.3-14.7kPa). Warm up to 129-130℃ , response 3h. Add half the amount of mother liquor to the reaction solution to dilute, add activated carbon to decolorize, and filter. The filtrate is cooled and crystallized in 10 ℃filter left and right, and the filter cake is washed with crude mother liquor to obtain crude isoniazid. Then it is recrystallized, activated carbon decolorized, filtered, and dried to obtain the finished product. Yield 90%.
Purpose
For biochemical research. High performance liquid chromatography fluorescence assay△4-3-Sterolones. pharmaceutical industry.
This product is used as the first choice anti-tuberculosis drug with high efficiency, low toxicity and low price. It is also used as electroplating additive and pharmaceutical intermediate. Isoniazid methane sulfonic acid can be produced by condensing nicotinamide and formaldehyde sodium bisulfite [13447-95-5]. Condenses with formaldehyde to form diisoniazid. This condensation reaction is more soluble and easier to proceed. Add formaldehyde to the isoniazid aqueous solution. mso-bidi-font-family: 宋体; mso-font-kerning: 1.0pt; mso-ansi-language: EN-US; mso-fareast-language: ZH-CN; mso-bidi-language: AR-SA “>℃Just wait about half an hour. The condensation of isoniazid and vanillic acid is also carried out in aqueous solution, and the reaction temperature is 95℃left and right, producing isoniazone. These isoniazid derivatives are anti-tuberculosis drugs. Among the isoniazids, isoniazid has the best effect. Isoniazid has a weaker effect on tuberculosis bacteria than isoniazid, but is less toxic to the nervous system and liver than isoniazid.
ly: ‘Times New Roman’; mso-font-kerning: 1.0pt; mso-ansi-language: EN-US; mso-fareast-language: ZH-CN; mso-bidi-language: AR-SA”>Left and right reactions It only takes half an hour. The condensation of isoniazid and vanillic acid is also carried out in aqueous solution, and the reaction temperature is 95℃left and right, producing isoniazid. These isoniazid derivatives are anti-tuberculosis drugs . Among the isoniazids, isoniazid has the best effect. Isoniazid has a weaker effect on tuberculosis bacteria than isoniazid, but its toxicity to the nervous system and liver is lighter than isoniazid.

 微信扫一扫打赏
微信扫一扫打赏

