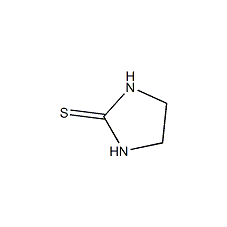
Structural formula
| Business number | 02B5 |
|---|---|
| Molecular formula | C3H6N2S |
| Molecular weight | 102.16 |
| label |
N,N’-Diethylenethiourea, Ethylene Thiourea, Ethylene Thiourea, Ethylene Thiourea, Tetrahydroimidazole-2-thione, 2-Thioimidazoline, Acid copper plating brightener N, Accelerator NA, ethylenethiourea, 2-imidazolidin ethione, 2-mercaptoimidazoline, 2-Thioxoimidazolidine, N,N’-Ethylenethiourea, accelerator NA, Imidazoline vulcanization accelerator, Auxiliary brightener for sulfate copper plating |
Numbering system
CAS number:96-45-7
MDL number:MFCD00005276
EINECS number:202-506-9
RTECS number:NI9625000
BRN number:106275
PubChem number:24869052
Physical property data
1. Properties: white needle-like crystals
2. Density (g/mL, 20℃): 1.41~1.45
3. Relative vapor density (g/mL, Air=1): Undetermined
4. Melting point (ºC): 203~204
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, kPa): Not determined
7. Refractive index: Undetermined
8. Flash point (ºC): 252
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg,ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature ( ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Soluble in alcohol, ethylene glycol and pyridine, insoluble in ether, benzene, chloroform and petroleum ether.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 28.30
2, Molar volume (cm3/mol): 79.9
3, Isotonic specific volume (90.2K): 223.6
4. Surface tension (dyne/cm): 61.1
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 11.21
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 56.2
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 63.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
It is stable under normal temperature and pressure.
Storage method
Packed in wooden barrels or woven bags and stored in dry warehouses. Pay attention to moisture-proof and keep away from fire sources (this product is flammable).
Synthesis method
1. In the glass-lined reaction pot, add water and ethylenediamine, lower the temperature by 20°C, add carbon disulfide, control it at 35-40°C, react for 4 hours, raise the temperature to recover the carbon disulfide, and generate vinyl dithiocarbamic acid Salt. Cool the vinyldithiocarbamate to below 50°C, add hydrochloric acid, release hydrogen sulfide when the temperature rises, and cyclize to generate ethylene thiourea. During the cyclization reaction, acetic acid can also be used instead of hydrochloric acid for the reaction. The crude ethylene thiourea obtained is dissolved in boiling water, filtered, cooled to precipitate crystals, and dehydrated, dried, and pulverized to obtain the finished product. (kg/ton) Ethylenediamine (70%) 740 Carbon disulfide (95%) 1250.
2. Put 24 kg of ethylenediamine, 48 kg of industrial alcohol, and 60 kg of distilled water into the reaction kettle in sequence. While stirring, slowly add 32 kg CS2, and control the temperature at around 60°C during the dropwise addition. After adding CS2, raise the temperature to 100 ℃ and reflux for 1 hour. Then add 3.6 kg concentrated hydrochloric acid and reflux for 9 to 10 hours. Crystallizes on cooling. The product was obtained by suction filtration, washing and drying with acetone. The yield is about 80% to 85%.
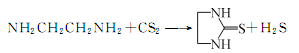
3. Using water as the reaction medium, carbon disulfide and ethylenediamine as raw materials to prepare ethylene thiourea (accelerator NA-22 or ETU), the reaction is carried out in two steps.
① Carbon disulfide reacts with ethylenediamine under the action of water to form the intermediate product ethylamine amino acid:
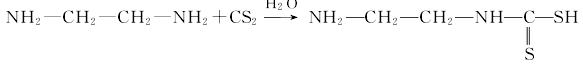
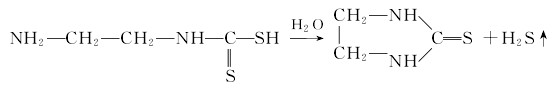
②The second step is the cyclization of ethylamino acid under the action of water to obtain the final product ethylene
Thiocarbamide:
Purpose
1. Used as an accelerator for chloroprene rubber, chlorosulfonated polyethylene rubber, chlorohydrin rubber, and polyacrylate rubber. Used as copper plating brightener. 2. This product is an imidazoline vulcanization accelerator, which can be used in various types of chloroprene rubber, chlorohydrin rubber, chlorosulfonated polyethylene rubber, polyacrylate rubber, etc. It is especially suitable for use as chloroprene rubber in non-vulcanized systems. safety accelerator. Usually below 100~500℃, by selecting an appropriate amount of blending system, you can achieve rapid and optimal vulcanization with safe operation. Its vulcanized products have high tensile strength and small permanent compression deformation. When used in the non-vulcanized system of W-type (i.e. 54-1 type) chloroprene rubber, the effect is remarkable. It is usually used together with zinc oxide and magnesium oxide and is mainly used in industrial products. It is easy to disperse in rubber materials, does not pollute, and does not change color. The dosage in general products is 0.25 to 1.5 parts.
3. This product is also an auxiliary brightener for sulfuric acid copper plating. This product is also used as an intermediate for fine chemicals in the manufacture of antioxidants, insecticides, fungicides, dyes, pharmaceuticals and synthetic resins. 4.Used as copper plating brightener, often used in conjunction with copper plating brightener M, etc.

 微信扫一扫打赏
微信扫一扫打赏

