Background and overview[1]
p-Nitrobenzenemethanesulfonyl fluoride is a type of sulfonyl fluoride compound. It is not only an important electrophile in organic synthesis, but also can perform addition reactions with many nucleophiles. It is also an important Free radical fluorination reagents. Recently, Doyle et al. used pyridinesulfonyl fluoride as a fluorination reagent to fluoride fatty alcohols (J. Am. Chem. Soc. 2015, 137, 9571). More importantly, it widely appears in many biologically active molecules and is also widely used as a labeling reagent for 18F, and a series of exciting results have been achieved.
Preparation method[1]
CN201510613062.4 provides a preparation method that uses sulfonyl hydrazide and fluorine reagent as starting materials for the reaction, and is easy to realize large-scale production of sulfonyl fluoride compounds. This method has mild conditions, is well compatible with water and air, does not require the addition of any catalyst, and only requires stirring and heating in the solvent to obtain various substituted sulfonyl fluoride compounds with high yields. The details are as follows:
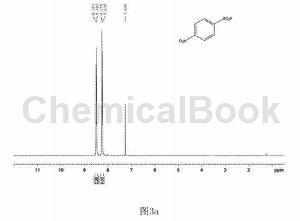
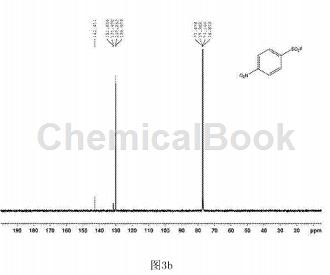
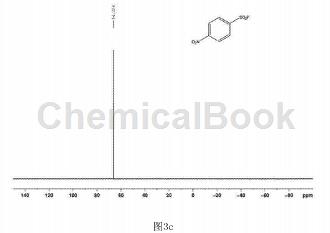
In a clean and dry 10 ml Schlenk reaction tube, add 54.3 mg of p-nitrobenzenesulfonyl hydrazide and 2.2.2 octane of 1-chloromethyl-4-fluoro-1,4-diazobicyclo in order. 123 mg of alkyl bis(tetrafluoroborate) was used, and 2 ml of water was used as the reaction solvent, and the reaction was stirred at 80°C for 18 hours. After the reaction is completed, extract by adding ethyl acetate, spin the upper organic phase directly to dryness, dissolve it with a small amount of petroleum ether and ethyl acetate (volume ratio is 30:1), and pass through a short silica gel column for column separation to obtain 34.7 mg of white solid. , yield 72%. The hydrogen NMR spectrum of the product is shown in Figure 3a, the carbon NMR spectrum is shown in Figure 3b, and the fluorine NMR spectrum is shown in Figure 3c. It can be confirmed from the spectrum that the obtained product is p-nitrobenzenesulfonyl fluoride.
Main reference materials
[1] CN201510613062.4 A kind of sulfonyl fluoridePreparation methods of compounds

 微信扫一扫打赏
微信扫一扫打赏

