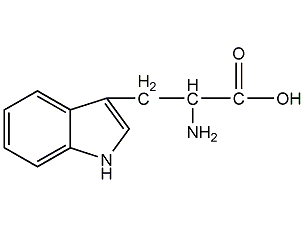
Structural formula
| Business number | 01HB |
|---|---|
| Molecular formula | C11H12N2O2 |
| Molecular weight | 204.23 |
| label |
L-2-amino-3-indolylpropionic acid, L-Aminoindolepropionic acid, L-trypsin amino acid, β-INDOLYLALANINE, (S)-2-Amino-3-indolylpropanoic acid, L-β-Indolylalanine, L-a-Aminoindole-3-propionic acid, amino acid drugs, intermediates, Biochemical reagents |
Numbering system
CAS number:73-22-3
MDL number:MFCD00064340
EINECS number:200-795-6
RTECS number:YN6130000
BRN number:86197
PubChem number:24278135
Physical property data
1. Characteristics: There are three isomers. The levorotatory body is a flaky crystal, tasteless.
2. Density (g/mL, 25/4℃): 1.362
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 289-290 (dec.)(lit.)
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2 kPa): Uncertain
7. Refractive index: -32 ° (C=1, H2O)
8. Flash point (ºC): Uncertain
p>
9. Specific optical rotation (º): -31.1 º (c=1, H20)
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25 ºC): Uncertain
12. Saturated vapor pressure (kPa, 60 ºC): Uncertain
13. Heat of combustion (KJ/ mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Oil and water ( Log value of the partition coefficient (octanol/water): Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/ V): Uncertain
19. Solubility: Slightly soluble in water (1.14%, 25℃) and ethanol. Soluble in dilute acid or alkali (20% NH3: 0.1 g/mL at 20 °C, clear, colorless), insoluble in chloroform and ether
Toxicological data
None
Ecological data
None
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 57.76
2. Molar volume (cm3/mol): 149.8
3. Isotonic specific volume (90.2K): 435.3
4. Surface tension (dyne/cm): 71.1
5. Polarizability (10-24cm3): 22.90
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 79.1
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 245
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 1
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Long-term exposure will cause coloring. A small amount of indole is produced when heated with water. A large amount of indole is produced when heated in the presence of sodium hydroxide or copper sulfate. It is more stable when heated with acid in a dark place. It is easily decomposed when coexisting with other amino acids, sugars and aldehydes.
2. Exist in burley tobacco leaves and smoke.
Storage method
Stored in a sealed, cool, dry and dark place
Synthesis method
1. Chemical synthesis
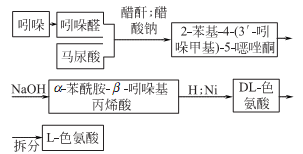
2.Fermentation method of adding precursors

3 .Direct fermentation method

4. It can be refined from casein through alkaline hydrolysis, or synthesized from β-indole aldehyde and hippuric acid.
Purpose
1. Nutritional and biochemical research. Prepare tissue culture medium.
2.Amino acid drugs. For amino acid infusion. Often combined with iron supplements, vitamins, etc. Used in combination with vitamin B6 to improve depression and prevent and treat pellagra; used as an insomnia sedative in combination with L-dopa to treat Parkinson’s disease; also used in vitamin B6 deficiency tests.

 微信扫一扫打赏
微信扫一扫打赏

