Background and overview[1]
3,4-Difluorobenzamide can be used as a pharmaceutical synthesis intermediate. If 3,4-difluorobenzamide is inhaled, move the patient to fresh air; if the skin comes into contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel uncomfortable; if the eye contact If exposed to sunlight, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation[1]
The preparation of 3,4-difluorobenzamide is as follows:
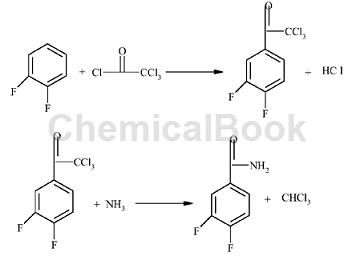
1) Add 228.4 kg (2.0 kmol) of 1,2-difluorobenzene, 382.4 kg (2.2 kmol) of trichloroacetyl chloride and 1600 kg of dichloroethane into the dry reactor, stir to dissolve, and add 220 kg of anhydrous zinc chloride. A large amount of gas escapes. Control the reaction temperature in an ice water bath to 25-30°C. After the raw materials react completely, pour the reaction solution into ice water with stirring. After layered washing, 2093 kg containing 3 , 493.0 kg (1.9 kmol) dichloroethane solution of 4-difluoro-(α,α,α-trichloroacetyl)benzene, yield 95%.
2) Add 2093 kg of dichloroethane solution containing 493.0 kg (1.9 kmol) of 3,4-difluoro-(α,α,α-trichloroacetyl)benzene into another reactor, stir Ventilate ammonia and control the reaction temperature to 20-30°C. A white solid will precipitate. After the raw material reaction is complete, filter and wash, and dry to obtain 289.6 kilograms (1.843 kmol) of 3,4-difluorobenzamide, with a yield of 97%.
Apply[1]
3,4-Difluorobenzamide can be used as a pharmaceutical synthesis intermediate. If the following reaction occurs:
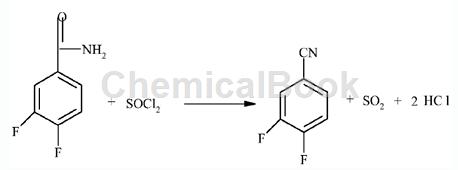
Add 289.6 kilograms (1.843 kmol) of 3,4-difluorobenzamide and 800 kilograms of dichloroethane solution into the dry reactor, and add 241.2 kilograms (2.02 kmol) of dichloroethane and the catalyst DMFI. .5 kg, react for 5 hours at 50°C with stirring. After the raw material reaction is complete, pour the reaction solution into ice water with stirring, and desolubilize in layers to obtain 3,4-difluorobenzene with a gas spectrum qualitative content of 96%. The crude nitrile product was 259.1 kilograms (1.788 kmol), and the yield was 97%. The crude product was distilled to obtain 248.6 kilograms (1.77 kmol) of 3,4-difluorobenzonitrile with a gas spectrum qualitative content of 99%, and the distillation yield was 99%.
Main reference materials
[1`] (CN103709071) Preparation method of 3,4-difluorobenzonitrile

 微信扫一扫打赏
微信扫一扫打赏

