Background and overview[1][2]
3-Butyl-1-phthalimide is an amine derivative and can be used as a pharmaceutical synthesis intermediate.
Preparation[1]
The preparation of 3-butyl-1-phthalimide is as follows:
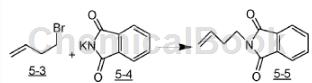
The specific steps are as follows: Add potassium phthalimide (5-4, 25g, 133mmol) and the mixture was stirred for 18 hours. hours at 70°C. After cooling to room temperature, the mixture was diluted with diethyl ether, washed with water and brine, dried over MgSO4, and concentrated to give 3-butyl-1-phthalimide as a white solid.
1HMR (400MHz, CDCl3): δ7.85 (m, 2H), 7.72 (m, 2H), 5.82 (m, 1H), 5.08 (m, 2H), 3.77 (t, 2H, J=7Hz) ,2.44(m,2H).
Apply[1]
3-Butyl-1-phthalimide can be used as a pharmaceutical synthesis intermediate. If the following reaction occurs:
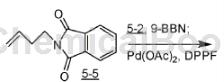
Step A: 5-(5-bromo-pyridin-2-yl)-valerate ethyl ester (5-2)
To a stirred solution of ethyl-1-pentenoic acid (10 g, 78 mmol) in degassed THF was added dropwise a solution of 9-BBN (187 mL, 0.5 M) in THF and the mixture was stirred at ambient temperature for 18 hours. , get 5J. Add K2CO3 (18.4g, 133mmol) and 2,5-dibromopyridine (18.5g, 78mmol), then premix and age (70°C, 30 minutes) the suspension of Pd(OAc)2 (2.0g, 8.9). (mmol) and DPPF (5.4 g, 9.8 mmol) were added to degassed DMF (80 mL). The resulting mixture was stirred at 70°C for 18 hours, cooled, diluted with ethyl acetate, washed with water and brine, dried over MgSO4 and concentrated. To the stirred residue dissolved in THF (400 mL) were added water (150 mL) and NaHCO3 (33g), and after 10 minutes, NaBO3*H2O (48g) was added. After stirring vigorously for 30 minutes, the mixture was diluted with ethyl acetateRelease, wash with water and brine, dry over MgSO4 and concentrate to oil. Chromatography of the residue on silica gel (10-20% EtOAc/hexanes) afforded 5-2 as a colorless oil.
1HMR (400MHz, CDC13): δ8.57 (s, 1H), 7.70 (m, 1H), 7.05 (d, 1H, J=8Hz), 4.15 (q, 2H, J=6Hz), 2.77 ( t, 2H, J=7Hz), 2.34 (t, 2H, J=7Hz), 1.7 (m, 4H), 1.26 (t, 3H, J=6Hz).
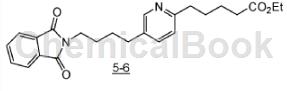
Step B: 5-{5-[4-(L3-dioxo-3,4-dihydro-isoindol-2-yl)-butyl-pyridin-2-yl|-ethyl valerate Esters (5-6)
To a stirred solution of 3-butyl-1-phthalimide (4.23 g, 21 mmol) in degassed THF (20 mL) at 0 °C, 9-BBN (50.4 mL of 0.5 M THF solution, 25.2 mmol) and this mixture. Stir at ambient temperature for 18 hours. Add K2CO3 (5.0g, 35.8mmol) and 5^2 (5.0g, 17.4mmol), then premix and age (70°C, 30 minutes) Suspension of Pd(OAc)2 (0.54g, 2.4mmol). and DPPF (1.45 g, 2.6 mmol) in degassed DMF (20 mL). The resulting mixture was stirred at 70°C for 18 hours, cooled, diluted with ethyl acetate, washed with water and brine, dried over MgSO4 and concentrated. To the stirred residue dissolved in THF (200 mL) were added water (75 mL) and NaHCO3 (16.5g). After 10 minutes, NaBO3*H2O (24g). After stirring vigorously for 30 minutes, the mixture was diluted with ethyl acetate, washed with water and brine, dried over MgSO4, and concentrated to an oil. Chromatography of the residue on silica gel (20-40% EtOAc/hexanes) afforded 5-6 as a yellow solid.
Main reference materials
[1](WO2016154369)COMPOSITIONANDMETHODSFORTREATINGCHRONICKIDNEYDISEASE

 微信扫一扫打赏
微信扫一扫打赏

