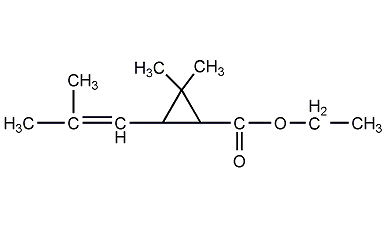
Structural formula
| Business number | 02C4 |
|---|---|
| Molecular formula | C12H20O2 |
| Molecular weight | 196 |
| label |
None yet |
Numbering system
CAS number:97-41-6
MDL number:MFCD00001304
EINECS number:202-579-7
RTECS number:GZ1727000
BRN number:2045740
PubChem number:24902026
Physical property data
1. Properties: colorless or light yellow transparent liquid.
2. Density (g/mL, 20℃): 0.906
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 178~179
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 1.33kPa): 112
p>
7. Refractive index: 1.459~1.461
8. Flash point (ºC): 84
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 20.2ºC): Not determined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Insoluble in water, soluble in ethanol, methanol, acetone , benzene, toluene and other organic solvents.
Toxicological data
1. Acute toxicity: Rat oral LD50: 2600mg/kg; Rat inhalation LC: >1600mg/m3; Mouse oral LD50: 2600mg/kg; Mouse inhalation LC: >1600mg/m3; Mouse skin contact LD50 :>5mg/kg; Guinea pig oral LD50: 1900mg/kg; Mammalian inhalation LC50: >1600mg/m3; 2. Mutagenicity: Gene conversion and mitotic recombination test: Saccharomyces cerevisiae, 1pph;
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 58.96
2. Molar volume (cm3/mol): 197.9
3. Isotonic specific volume (90.2K ): 482.6
4. Surface tension (dyne/cm): 35.3
5. Polarizability (10-24cm3)��23.37
Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 260
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 2
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
None yet
Synthesis method
Diazoacetate method
Add 2,5-dimethyl-2,4-hexadiene into the reaction bottle, use copper powder with a diene dosage of 59/6 as a catalyst, and then add a little Hydroquinone is an antioxidant. Heat it to 120°C under nitrogen. Under rapid stirring, drop the methyl diazoacetate solution. N2 will be released during the dropwise addition. Control the reaction speed and add dropwise. After completion, continue stirring for 0.5 hours, cool, and distill away the excess diene. Then increase the vacuum degree to evaporate the methyl chrysanthemate. The recovered diene can be recycled. Use ethyl diazoacetate instead of methyl diazoacetate to obtain ethyl chrysanthemate.
Purpose
Mainly used as key intermediates for pesticides such as allethrin, penmethrin, phenethrin, and proparthrin.

 微信扫一扫打赏
微信扫一扫打赏

