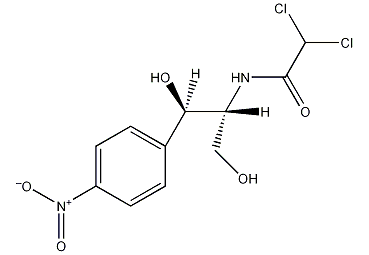
Structural formula
| Business number | 017V |
|---|---|
| Molecular formula | C11H12Cl2N2O5 |
| Molecular weight | 323.14 |
| label |
levomycin, 2,2-Dichloro-N-[2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl]acetamide, fungicides, Genetic engineering research reagents |
Numbering system
CAS number:56-75-7
MDL number:MFCD00078159
EINECS number:200-287-4
RTECS number:AB6825000
BRN number:2225532
PubChem number:24892250
Physical property data
1. Character: white or slightly yellow Green needle-like crystals.
2. Density ( g/mL,25/4℃) : 1.474
3. Relative vapor density (g/mL,AIR=1): Undetermined
4. Melting point ( ºC): 150.5-151.5℃(149.7-150.7℃). Can sublimate under high vacuum.
5. Boiling point ( ºC,Normal pressure): Undetermined
6. Boiling point ( ºC,5.2kPa): Undetermined
7. Refractive Index: Undetermined
8. Flashpoint (ºC): Undetermined
9. Specific optical rotation (º): 19.5° (c=6, EtOH) .
10. Autoignition point or ignition temperature (ºC): Not OK
11. Vapor pressure (kPa,25ºC): Undetermined
12.):208.8
3. isotonic specific volume (90.2K):595.5
4. Surface Tension (dyne/cm):66.1
5. Polarizability(10-24cm3): 28.76
Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: 2
6. Topological molecule polar surface area 115
7. Number of heavy atoms: 20
8. Surface charge: 0
9. Complexity: 342
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 2
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be stored in a sealed, cool place away from light.
Synthesis method
Countries around the world have conducted a large number of studies on the production methods of chloramphenicol, which can be summarized as follows: (1) Yes Nitroacetophenone method; (2) Styrene method; (3) Cinnamyl alcohol method; (4) p-nitrocinnamyl alcohol method; (5) p-nitrobenzaldehyde method. my country adopts the p-nitroacetophenone method, which obtains chloramphenicol from ethylbenzene through nitration; oxidation; bromination; salt formation; hydrolysis; acetylation; addition; reduction; decomposition; separation; and dichloroacetylation. .
Purpose
It is a broad-spectrum antibacterial antibiotic and is used to treat typhoid fever and paratyphoid fever It is the drug of choice and one of the most effective drugs in the treatment of anaerobic bacterial infections. It is also used in the treatment of various infectious diseases caused by sensitive microorganisms. Due to serious adverse reactions, it is used less and less.
FAMILY: Arial; mso-fareast-font-family: 宋体; mso-ansi-language: EN-US; mso-fareast-language: ZH-CN; mso-bidi-language: AR-SA”>2 ) styrene method; (3) Cinnamyl alcohol method; (4) p-nitrocinnamic alcohol method; (5) p-Nitrobenzaldehyde method. my country adopts the p-nitroacetophenone method, which obtains chloramphenicol from ethylbenzene through nitration; oxidation; bromination; salt formation; hydrolysis; acetylation; addition; reduction; decomposition; separation; and dichloroacetylation. .
Purpose
It is a broad-spectrum antibacterial antibiotic and is used to treat typhoid fever and paratyphoid fever It is the drug of choice and one of the most effective drugs in the treatment of anaerobic bacterial infections. It is also used in the treatment of various infectious diseases caused by sensitive microorganisms. Due to serious adverse reactions, it is used less and less.

 微信扫一扫打赏
微信扫一扫打赏

