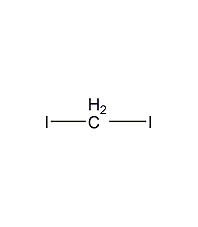
Structural formula
| Business number | 01J0 |
|---|---|
| Molecular formula | CH2I2 |
| Molecular weight | 267.84 |
| label |
methylene iodide, Methine iodide, methylene diiodide, methylene iodide, Methylene iodide, Methylene diodide, Aliphatic halogenated derivatives |
Numbering system
CAS number:75-11-6
MDL number:MFCD00001079
EINECS number:200-841-5
RTECS number:PA8575000
BRN number:1696892
PubChem number:24849735
Physical property data
1. Properties: colorless clear to light yellow liquid. [14]
2. Melting point (℃): 5~6[15]
3. Boiling point (℃) : 181 (decomposition) [16]
4. Relative density (water = 1): 3.32[17]
5. Relative vapor density (air = 1): 9.25[18]
6. Heat of combustion (kJ/mol): -745.7[19]
7. Critical pressure (MPa): 5.47[20]
8. Octanol/water partition coefficient: 2.3[21]
9. Flash point (℃): 110[22]
10. Solubility: insoluble in water, soluble in ethanol, Most organic solvents such as ether, benzene, and chloroform. [23]
11. Refractive index at room temperature (n20): 1.7411
12. Refractive index at room temperature (n25): 1.7380
13. Solubility parameter (J·cm-3)0.5: 24.055
14. van der Waals area (cm2·mol-1): 6.430×109
15. van der Waals volume (cm3·mol-1): 50.930
16. Viscosity (10ºC): 3.35mPa. s
17. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): 67.8
18. Liquid phase standard hot melt (J ·mol-1·K-1): 135.5
19. The gas phase standard claims heat (enthalpy) (kJ·mol-1 ): 118.7
20. Gas phase standard entropy (J·mol-1·K-1): 309.50
21. Gas phase standard formation free energy (kJ·mol-1): 101.7
22. Gas phase standard hot melt (J·mol-1 sup>·K-1): 57.73
Toxicological data
1. Acute toxicity
Children’s oral LDLO: 2778 uL/kg
Rat abdominal LD50: 403mg/kg
Mouse abdominal LD50: 467mg /kg
Mouse subcutaneous LD50: 830mg/kg
2. Acute toxicity[24] LD50: 403mg/kg (rat oral); 830mg/kg (rat transdermal)
3. Irritation No data available
4. Mutagenicity [25] Microbial mutagenicity: Escherichia coli 3mg/dish.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
The source is crucial to the success or failure of the reaction. Zn/Cu, diethyl zinc, etc. can be used as sources of Zn for cyclopropanation reaction. Diiodomethane and samarium mercury or samarium iodide can be combined to obtain many different samarium-containing olefin cyclopropanation reagents, which can react with allyl alcohol and enol. α,β-unsaturated esters and α,β-unsaturated amides can also be combined with samarium catalysis Diiodomethane undergoes cyclopropanation reaction (Formula 3)[3]. The cyclopropanation reactions of zinc-containing reagents and samarium-containing reagents are directly affected by hydroxyl groups[4]. Treating diiodomethane with trialkyl aluminum (such as triisobutylaluminum) will also give the corresponding cyclopropane, which is a good complement to the zinc and samarium system. This reaction tends to react with independent alkenes, while It does not react with allyl alcohol (Formula 4)[5].
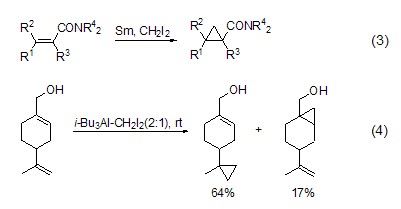
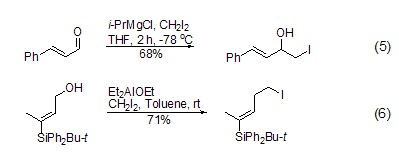
‘ICHNucleophilic addition of 2‘ Methyl iodide prepared from samarium metal [6] can react with aldehydes, ketones and enones, and magnesium reagents can also be used For this reaction (Equation 5)[7]. The aluminum reagent can also be used to replace the allyl alcohol hydroxyl group with iodomethyl, Et3Al, Et2AlCl, Et2AlOEt All can participate in this reaction (such as equation 6)[8].
|
(+)-trans-(2S,3S)-bis(diphenylphosphine)bicyclo[2.2.1]hept-5-ene |
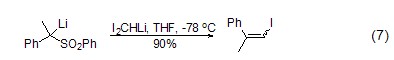
Nucleophilic addition of ‘I2CH’ CH2I2 deprotonates under the action of a base After oxidation, I2CHM derivatives are obtained. These compounds are more stable than the corresponding ICH2M and can react with many electrophiles[9]. Commonly used bases include Cy2NLi, NaHMDS, LiHMDS and LDA. Allyl iodide is synthesized using I2CHLi. First treat diiodomethane with LiHMDS, then add sulfone, and evaporate the water to obtain allyl iodide, but the selectivity is relatively poor (Formula 7)[9].
Free radical addition Addition of ICH2 fragments to α,β-unsaturated ketones in the presence of triethylborane Reaction to obtainγ-iodoketone (formula 8)[10]. The intermediate boron enolate can be either hydrolyzed or alkylated.

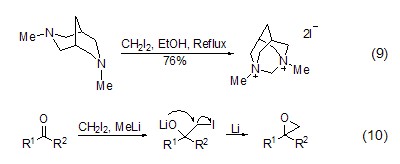
Alkylation reaction The application of diiodomethane in alkylation is limited. ClCH2I and ClCH2Br are more prone to alkylation reactions than diiodomethane, but , diiodomethane can be used in cycloalkylation reactions. Diamine can react with diiodomethane. Slowly adding diiodomethane solution to the diamine solution can obtain a higher yield (Formula 9)[11]. In the reaction with Pt as a catalyst, diiodomethane reacts with thiol to obtain dithiane[12]. In addition, the in-situ generation of iodomethyllithium in the presence of diiodomethane and alkyl lithium can easily and quickly convert many carbonyl compounds into epoxides (Formula 10)[13].
3. Used in organic synthesis and Separation of mixed minerals. [33]

 微信扫一扫打赏
微信扫一扫打赏

