Background and overview[1][2]
Ethyl 5-phenylisoxazole-3-carboxylate is an organic chemical intermediate that can be prepared from phenylacetylene or ethyl 2,4-dioxo-4-phenylbutyrate as starting materials. .
Preparation[1-2]
Method 1,
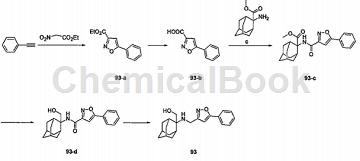
Phenylacetylene (20g, 0.2mol) and ethyl nitroacetate (66.4g, 0.5mol) were dissolved in 500ml of chloroform, and then 11.2g of triethylenediamine (DABCO, 0.1mol) was added. The solution was heated to 70°C for 72 hours. Cool to room temperature, add 200 ml of water to dilute, sodium bicarbonate aqueous solution (100 ml), and saturated saline (100 ml). The organic phase was collected, dried, and the solvent was removed. The crude product was purified by column chromatography (petroleum ether: ethyl acetate = 300:1) to obtain 30 grams of compound 93-a (yield 71%). 1HNMR (400MHz, CDCl3): δ1.41-1.45 (t, J=7.0Hz, 3H), 4.43-4.48( m, 2H), 6.91 (s, 1H), 7.46-7.49 (m, 3H), 7.78-7.81 (m, 2H).
Method 2,
The raw material 2,4-dioxo-4-phenylbutyric acid ethyl ester (1g, 4.54mmol) was dissolved in 20ml of absolute ethanol, hydroxylamine hydrochloride (0.63g, 9.08mmol) was added in batches, and the reaction was refluxed for 2 hours. After the reaction, pour the reaction solution into 50 ml of water, extract with ethyl acetate (20 ml × 3), combine the organic phases, wash with saturated brine (15 ml × 2), dry over anhydrous sodium sulfate, filter, and evaporate the solvent from the filtrate under reduced pressure. , 0.6g of white solid was obtained, melting point was 59-60°C, and yield was 60.7%. 1H NMR (300MHz, DMSO-d6) δ: 7.98, 7.96 (dd, J=1.31, 7.44Hz, 2H, ArH), 7.57-7.52 (m, 3H, ArH), 7.49 ( s, 1H, ArH), 4.40 (q, J=7.11Hz, 2H, -OCH2), 1.35 (t, J=7.11Hz, 3H, -CH3).
Apply[1]
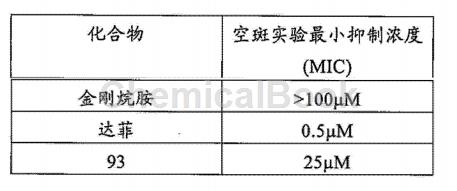
Can be used to prepare compound 93. Pre-culture MDCK cells to approximately 90% density using a 12-well plate. The WSN virus was diluted to a concentration of 50 PFU, and Example Compound 93 was added and mixed respectively. Amantadine and Tamiflu were used as control drugs at different concentrations according to the gradient (100 μM, 50 μM, 25 μM, 10 μM, 5 μM, 2.5 μM, 1 μM, 0.5 μM , 0.25 μM and 0.1 μM) were added to MDCK cells and incubated for 30 minutes. The incubated virus liquid was sucked out and mixed with agar and 2×DMEM. The mixture was then added to the well plate and incubated for about 48 hours. Add crystal violet for fixed staining. Select the minimum concentration in the concentration gradient that inhibits the appearance of plaques in the experiment as the minimum inhibitory concentration of the plaque experiment, which is used to quantitatively determine the virus-inhibiting effect of the compound. The results show that the inhibitory effect of compound 93 of the present invention on viral infection is much stronger than that of amantadine, and compound 93, Tamiflu and amantadine did not show any cytotoxic effect at 100 μM.
Main reference materials
[1] CN201410056606.7 Antiviral compounds, preparation methods and uses thereof
[2] CN201510432499.8 New biphenyl heterocyclic derivatives, their preparation methods and their use as drugs

 微信扫一扫打赏
微信扫一扫打赏

