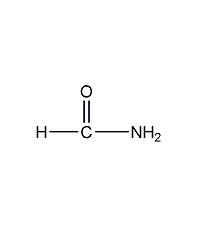
Structural formula
| Business number | 01J1 |
|---|---|
| Molecular formula | CH3NO |
| Molecular weight | 45.04 |
| label |
Aminoformaldehyde, Methanamide, Carbamaldehyde, Reagents for genetic engineering research, paper treatment agent, Softeners for the fiber industry, Softener for animal glue, Reaction solvents for organic synthesis |
Numbering system
CAS number:75-12-7
MDL number:MFCD00007941
EINECS number:200-842-0
RTECS number:LQ0525000
BRN number:505995
PubChem number:24894985
Physical property data
1. Properties: Colorless and transparent viscous liquid with a slight ammonia smell and hygroscopicity.
2. Boiling point (ºC, 101.3kPa, partially decomposed): 220, 70.5ºC (133.3pa)
3. Melting point (ºC): 2.55~3
4. Relative density (g/mL, 20/4ºC): 1.13339
5. Relative density (g/mL, 25/4ºC): 1.134
6. Relative steam Density (g/mL, air=1): 1.55
7. Refractive index (20ºC): 1.447
8. Refractive index (25ºC): 1.44682
9. Viscosity (mPa·s, 20ºC): 3.764
10. Viscosity (mPa·s, 25ºC): 3.302
11. Flash point (ºC, closed): 175
12. Flash point (ºC, open): 150
13. Fire point (ºC): >500
14. Heat of vaporization (KJ/mol, 25ºC): 65.021
15. Heat of fusion (KJ/mol): 6.699
16. Heat of formation (KJ/mol, 25ºC, liquid): -254.1
17. Heat of combustion (KJ/mol, 25ºC, liquid): 568.6
18. Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure): 2.39
19. Conductivity (S/m): <2×10-1
20. Solubility: Can be dissolved with water, alcohol, ethylene glycol, acetone, acetic acid, dihydrogen Miscible with alkane, glycerin, phenol, etc. But it is almost insoluble in aliphatic hydrocarbons, aromatic hydrocarbons, ethers, chlorinated hydrocarbons, chlorobenzene, nitrobenzene, etc.
Toxicological data
Formamide has an irritating effect on the skin and mucous membranes, can occasionally cause allergies, and can be absorbed by the skin. The oral lethal dose LD for rats is 7500 mg/kg. Rat oral LD50>4000mg/kg. Dermal toxicity in guinea pigs is LD50<5mL/kg and LD50 is 2539mg/kg.
Ecological data
None yet
Molecular structure data
1. Molar refractive indexal/day_100929/201009291018234204.gif” alt=”” />
Purpose
1. Formamide has active reactivity and special solubility. It can be used as a raw material for organic synthesis, paper treatment agent, softener in the fiber industry, softener for animal glue, and also used to determine the amino acid content in rice. Analytical reagents. In organic synthesis, it is mostly used in medicine, and it also has many uses in pesticides, dyes, pigments, spices, and auxiliaries. It is also an excellent organic solvent, mainly used in the spinning of acrylonitrile copolymers and ion exchange resins, as well as anti-static coating or conductive coating of plastic products. In addition, it is also used to separate chlorosilanes, purify oils, etc. Formamide can undergo a variety of reactions. In addition to the participation of three hydrogens in the reaction, it can also undergo dehydration, removal of CO, introduction of amino groups, introduction of acyl groups and cyclization reactions. Take Ringhe as an example. Diethyl malonate is cyclized with formamide to obtain the intermediate 4,6-dihydroxypyrimidine of vitamin B4. Anthranilic acid is cyclized with an amide to obtain quinazolone-4, an intermediate of antiarrhythmic croroline. 3-Amino-4-ethoxycarbonylpyrazole is cyclized with carboxamide to obtain the xanthine oxidase inhibitor allopurinol. . The anticancer drug ethyleneimine is obtained by cyclizing ethylenediaminetetraacetic acid with formamide. Methyl ethyl methoxymalonate is cyclized with formamide to obtain disodium 5-methoxy-4,6-dihydroxypyrimidine, an intermediate of sulfonamide drugs.
2. Since formamide can dissolve inorganic salts and proteins with high dielectric constants, it can be used in the electrolysis and electroplating industries, as well as as reaction solvents and refining solvents for organic synthesis. In addition, formamide is also used as a raw material for medicines, dyes, spices, etc., as a treatment agent for paper, as a softener in the fiber industry, and as a softener for animal glue.
3.Used as raw material for organic synthesis. Polar solvent for organic reactions. Liquid chromatography solvents and eluents.

 微信扫一扫打赏
微信扫一扫打赏

