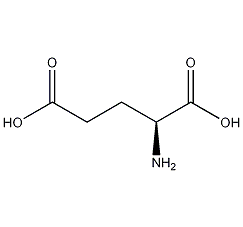
Structural formula
| Business number | 0180 |
|---|---|
| Molecular formula | C5H9NO4 |
| Molecular weight | 147.13 |
| label |
(S)-2-Aminoglutaric acid, glutamine, (S)-2-Aminoglutaric acid, L-Glutamic acid, (S)-(+)-Glutamic acid, Bitter remover, intermediates, Biochemical reagents |
Numbering system
CAS number:56-86-0
MDL number:MFCD00002634
EINECS number:200-293-7
RTECS number:LZ9700000
BRN number:1723801
PubChem number:24901609
Physical property data
1. Characteristics: L-glutamic acid is white or colorless scaly crystals and is slightly acidic. The racemate, DL-glutamic acid, is a colorless crystal.
2. Density (g/mL, 25/4℃): Racemic: 1.4601; Dextral, left-handed: 1.538.
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 160
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8 . Flash point (ºC): Undetermined
9. Specific rotation (º): [α]D22.4+31.4° (C=1.6mol/L hydrochloric acid)
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC ): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical Pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: racemateSlightly soluble in cold water, easily soluble in hot water, almost insoluble in ether, ethanol and acetone. The racemate is slightly soluble in ethanol, ether and petroleum ether.
Toxicological data
1. Acute toxicity: human oral TDLo: 71mg/kg; human intravenous TDLo: 117mg/kg; rat oral LD50: 36mg/kg; rabbit oral LD50: >2300mg/kg 2. Mutagenicity: sister chromatids exchangeTEST system:�Lymphocytes: 10mg/L
Ecological data
General remarks
Water hazard level 1 (German regulations) (self-assessment via list) This substance is slightly hazardous to water.
Do not allow undiluted or large amounts of product to come into contact with groundwater, waterways or sewage systems.
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 31.83
2. Molar volume (cm3/mol): 104.3
3. Isotonic specific volume (90.2K ): 301.0
4. Surface tension (dyne/cm): 69.2
5. Polarizability (10-24cm3): 12.62
Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 101
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 145
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 1
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. This product is non-toxic.
2.Odorless, with a slightly special and sour taste.
3. Exist in tobacco leaves and smoke.
Storage method
1. This product should be sealed and stored in a cool, dark place.
2. Packed in plastic bags, nylon bags or plastic woven bags, net weight 25kg. During storage and transportation, attention should be paid to moisture-proof, sun-proof and low-temperature storage.
Synthesis method
1. Glutamic acid can be produced by protein hydrolysis and synthesis, but fermentation is currently the main method for producing glutamic acid. The carbon source for fermentation to produce glutamic acid is hydrolyzed sugar or molasses from potato, corn, tapioca starch, coconut tree starch and other starches. It can also be acetic acid, liquid paraffin (C16 paraffin is best) and other petrochemical products. The carbon source is Nutrients that constitute the carbon framework and energy in microbial cells and metabolites. Nitrogen sources are ammonium salts, urea, etc. Nitrogen is the main element that constitutes bacterial cell proteins and nucleic acids. Nitrogen is also the main element that constitutes the glutamic acid amino group of fermentation products. Other auxiliary raw materials are inorganic salts, vitamins, etc. For example, microorganisms require appropriate phosphorus concentration, magnesium is an inorganic activator that stimulates bacterial growth, potassium salt promotes acid production, and corn steep liquor provides biotin and organic nitrogen sources. Various accelerators and additives are also included. The producing bacteria are Brevibacterium, Corynebacterium pekinensis, etc. In a large fermentation tank, ferment with aeration and stirring at a temperature of 30-34°C and a pH>7-8. After 30-40 hours of fermentation, remove bacteria and extract glutamic acid from the fermentation liquid. After refining, the finished product is obtained. In the above process Extraction using isoelectric point method, ion exchange method, hydrochloride method, direct concentration method (when acetic acid is used as raw material), etc. can also be used. The product produced by fermentation method is L-glutamic acid, with a content of more than 98%. Each ton of glutamic acid consumes 4000kg of starch (80%) and 25kg of bacteria. The advantage of the synthesis method is that it does not consume food, but the production process requires high pressure (about 20MPa), high temperature (above 120°C), and uses toxic raw materials. The equipment investment is twice as high as that of the fermentation method, and the racemic glutamic acid obtained needs to be further processed. Split, the production process is complex. Calculated based on the production of 1 ton of 99% sodium glutamate (MSG), the synthesis method consumes 640kg of acrylonitrile. When the annual output is more than 5,000t, the production cost is close to that of the acidification method.
2.Fermentation method

3. Chemical synthesis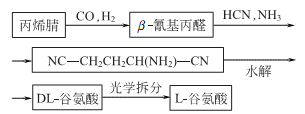
4.This product is mainly made from fermentation Produced by law. Molasses or starch is used as the raw material, Corynebacterium glutamicum or Pediococcus or Arthrobacter is used as the strain, and urea is used as the nitrogen source. Fermentation is carried out at 30 to 32°C. After the fermentation is completed, the bacteria are separated from the fermentation liquid. Use hydrochloric acid to adjust the pH value to 3.0, perform isoelectric point extraction, and obtain glutamic acid crystals after separation. The glutamic acid in the mother liquor is extracted with 732 ion exchange resin, crystallized, and dried to obtain the finished product.
5. Tobacco: BU, 22; FC, 21; L-isomer can be obtained from animal and plant proteins through hydrolysis, decolorization, concentration, and crystallization. It can also be produced from sugar or starch by fermentation. The racemate can be synthesized from acrylonitrile.
Purpose
1.L-Glutamic acid is mainly used in the production of MSG and spices, as well as as salt substitutes, nutritional supplements and biochemical reagents. L-glutamic acid itself can be used as a drug, participating in the metabolism of proteins and sugars in the brain, and promoting the oxidation process. This product combines with ammonia in the body to form non-toxic glutamine, which reduces blood ammonia and relieves the symptoms of hepatic coma. It is mainly used to treat hepatic coma and severe liver insufficiency, but the efficacy is not very satisfactory; combined with anti-epileptic drugs, it can still treat petit mal epilepsy and psychomotor seizures. Racemic glutamate is used in the production of drugs and as biochemical reagents.
2.Usually not used alone, but in conjunction with phenolic and quinone antioxidants to obtain good synergistic effects.
3.Glutamic acid is used as a complexing agent for electroless plating.
Amine can reduce blood ammonia and relieve symptoms of hepatic coma. It is mainly used to treat hepatic coma and severe liver insufficiency, but the efficacy is not very satisfactory; combined with anti-epileptic drugs, it can still treat petit mal epilepsy and psychomotor seizures. Racemic glutamate is used in the production of drugs and as biochemical reagents.
2.Usually not used alone, but in conjunction with phenolic and quinone antioxidants to obtain good synergistic effects.
3.Glutamic acid is used as a complexing agent for electroless plating.

 微信扫一扫打赏
微信扫一扫打赏

