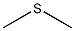
Structural formula
| Business number | 01J5 |
|---|---|
| Molecular formula | C2H6S |
| Molecular weight | 62.13 |
| label |
dimethyl sulfide, dimethyl sulfide, DMS, Dimethyl sulfide, 2-Thiapropane, Methyl sulphide, City gas smell tester, industrial purifier, paint release agent, Battery low temperature preservative, pesticide penetrant |
Numbering system
CAS number:75-18-3
MDL number:MFCD00008562
EINECS number:200-846-2
RTECS number:PV5075000
BRN number:1696847
PubChem number:24856582
Physical property data
1. Properties: colorless liquid with unpleasant odor. [1]
2. Melting point (℃): -98.3[2]
3. Boiling point (℃): 37.3[3]
4. Relative density (water = 1): 0.85[4]
5. Relative vapor Density (air=1): 2.14[5]
6. Saturated vapor pressure (kPa): 53.2 (20℃)[6]
7. Heat of combustion (kJ/mol): -1907.7[7]
8. Critical temperature (℃): 229[8]
9. Critical pressure (MPa): 5.69[9]
10. Octanol/water partition coefficient: 0.92 [10]
11. Flash point (℃): <-17.7[11]
12. Ignition temperature (℃) :206[12]
13. Explosion upper limit (%): 19.7[13]
14. Explosion lower limit (%) %): 2.2[14]
15. Solubility: Insoluble in water, soluble in most organic solvents such as ethanol and ether. [15]
16. Viscosity (mPa·s, 20ºC): 0.289
17. Viscosity (mPa·s, 25ºC): 0.279
18. Ignition point (ºC): 206.1
19. Heat of evaporation (KJ/mol, 25ºC): 27.61
20. Heat of evaporation (KJ/mol, b.p. ): 27.0174
21. Heat of fusion (KJ/mol): 7.990
22. Heat of formation (KJ/mol, 25ºC): -65.48
23 .Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure): 1.17
24. Boiling point rising constant: 1.85
Toxicological data
1. Acute toxicity:
Rat caliber LD50: 3300mg/kg; rat inhalation LCLO: 40250ppm;
Mouse caliber LD50: 3700mg/kg; mouse inhalation LCLO: 31620 ug/m3;
Mouse abdominal LD50: 8mg/kg; Rabbit skin LD50: >5mg/kg;
Mammal LD50: 1950mg/kg;
2. Other multiple dose toxicity data:
Rat caliber TDLO: 21mg/kg/70D-I; Rabbit caliber TDLO: 24500mg/kg/14W-I;
3. Acute toxicity[16]
LD50: 535mg/kg (rat oral)
LC50: 102235mg /m3(Rat inhalation)
4. Irritation[17]
Rabbit transdermal: 500mg (24h),Mild irritation.
Rabbit eye: 259μg (24h), severe irritation.
Ecological data
1. Ecotoxicity[18] LC50: 7.5~15ppm (fish)
2. Biodegradability No data yet
3. Non-biodegradability[19] In the air, when the hydroxyl radical concentration is 5.00×10 At 5pcs/cm3, the degradation half-life is 3.5d (theoretical).
The half-life of photooxidation is 8 hours.
Molecular structure data
1. Molar refractive index: 19.31
2. Molar volume (cm3/mol): 75.5
3. Isotonic specific volume (90.2K ): 162.3
4. Surface tension (dyne/cm): 21.3
5. Polarizability (10-24cm3): 7.65
Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.9
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 25.3
7. Number of heavy atoms: 3
8. Surface charge: 0
9. Complexity: 2.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: It can form addition compounds with halogens, metal halides, etc. Or react with alkyl halides to form sulfonium compounds. During oxidation, sulfoxide is generated, and continued oxidation generates sulfone. It is easy to burn and explode when exposed to open flames or high heat. Thermal decomposition produces toxic sulfide fumes.
2. This product is poisonous. Low-concentration dimethyl sulfide vapor generally causes nausea and loss of appetite, while high-concentration vapor has a paralyzing effect on the central nervous system. During the production process, it must be sealed to prevent running, popping, dripping and leaking. Ventilation should be strengthened at the production site, and operators should wear protective equipment. In the event of poisoning, you should move to a place with fresh air and seek medical treatment.
3. Stability[20] Stable
4. Incompatible substances[21] Strong oxidants, alkalis, ammonia
5. Conditions to avoid contact [22] Heat
6. Polymerization hazard[23] No polymerization
7. Decomposition products[24] Sulfide
Storage method
Storage Precautions[25] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 29℃. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, alkalis, and ammonia, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Refining method: Use mercuric chloride and methyl sulfide to form a complex for refining: add 1 mol of mercuric chloride to 1250 mL of ethanol, and then add 0.67 mol of methyl sulfide alcohol solution to this solution. The resulting solid is recrystallized to obtain complex crystals with a certain melting point and a structure of 2(CH3)2S·HgCl2. Dissolve 250 mL of concentrated hydrochloric acid in 780 mL of water, add 500 g of the refined complex, and heat to separate the methyl sulfide. After washing with water and drying with calcium chloride, the purity can reach 99.995% (mol).

Purpose
1. This product is a solvent and an intermediate for the production of dimethyl sulfoxide, methionine and pesticides. It can be used as a solvent for organic compounds, resins, inorganic compounds, polymerization reactions and cyanide reactions. Used as analytical reagent, polyacrylonitrile and other synthetic fiber spinning and hydraulic oil. It can also be used as an odorizer for city gas, industrial purifier, paint release agent, battery low-temperature preservative, pesticide penetrant, etc. Used topically in hematological medicines, phytopathology and nutrition.
2. Used as solvent and catalyst for most inorganic substances. [26]

 微信扫一扫打赏
微信扫一扫打赏

