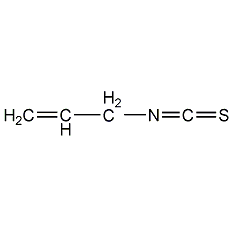
Structural formula
| Business number | 0189 |
|---|---|
| Molecular formula | C4H5NS |
| Molecular weight | 99.16 |
| label |
3-isothiocyanato-1-propene; artificial mustard oil, 3-Different thiocyanomethylthio-1-acrylamide, Artificial mustard oil, Allyl isothiocyananate, Isothiocyanic acid allyl ester, iso-Thiocyanic acid allyl ester, artificial flavors |
Numbering system
CAS number:57-06-7
MDL number:MFCD00004822
EINECS number:200-309-2
RTECS number:NX8225000
BRN number:773748
PubChem number:24862709
Physical property data
1. Properties: colorless or light yellow oily liquid with pungent odor. [1]
2. Melting point (℃): -80[2]
3. Boiling point (℃): 150.7[3]
4. Relative density (water = 1): 1.01[4]
5. Relative vapor Density (air=1): 3.41[5]
6. Saturated vapor pressure (kPa): 1.33 (38.3℃)[6]
7. Octanol/water partition coefficient: 2.11[7]
8. Flash point (℃): 46 (CC)[8 ]
9. Solubility: Slightly soluble in water, miscible in most organic solvents such as ethanol, ether, carbon disulfide, etc. [9]
10. Refractive index (n20/D): 1.527-1.531
Toxicological data
1. Acute toxicity[10] LD50: 112mg/kg (rat oral); 88mg/kg (rabbit dermal)
2. Irritation [11] Rabbit transdermal; 2mg, causes irritation.
3. Mutagenicity [12] Microbial mutagenicity: Salmonella typhimurium 100μg/dish. Mammalian somatic mutations: mouse lymphocytes 400μg/L. Cytogenetic analysis: Hamster ovary 5mg/L. Sister chromatid exchange: Hamster ovary 160μg/L. DNA damage: human ascites tumor 50μmol/L (24h)
4. Carcinogenicity [13] IARC Carcinogenicity Comment: G3, for humans and There is insufficient evidence of carcinogenicity in animals.
5. Others[14] Rat subcutaneous minimum toxic dose (TDLo): 100mg/kg (gestation 8~9d), causing embryotoxicity (embryonic development retardation)
Ecological data
1. Ecotoxicity[15] LC50: 0.0856mg/L (96h) (fathead minnow, dynamic)
2. Biodegradability No data available
3. Non-biodegradability No data available
Molecular structure data
1. Molar refractive index: 31.17
2. Molar volume (cm3/mol): 108.8
3. Isotonic specific volume (90.2K ): 249.8
4. Surface tension (dyne/cm): 27.7
5. Polarizability (10-24cm3��:12.35
Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.4
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
p>
4. Number of rotatable chemical bonds: 2
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 12.4
p>
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 81.5
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Colorless to light yellow transparent oily liquid, the color gradually turns darker during storage. It has a strong mustard-like pungent odor and spicy taste. No optical activity. Miscible in ethanol, ether and carbon disulfide. It has anti-fungal and bactericidal properties.
2. Stability[16] Stable
3. Incompatible substances[17] Water, alcohols, strong bases, amines, acids, strong oxidants
4. Conditions to avoid contact[18] Humid air
5. Polymerization hazard[19] Polymerization
6. Decomposition products[20] Hydrogen cyanide, sulfide
Storage method
Storage Precautions[21] Stored in a cool, dry and well-ventilated special warehouse, and implement “two people to send and receive, two people to keep” “system. Keep away from fire and heat sources. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, acids, alkalis, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Dissolve allyl iodide or allyl bromide and potassium thiocyanate in ethanol and heat. After the potassium halide is completely precipitated, add water to precipitate allyl isothiocyanate, and distill and refine. The actual yield is 65%-70% of the theoretical value.
Purpose
1. Used for spices. In the manufacture of military uniform poison gas, ointment is used as a counter-irritant. GB 2760–1996 stipulates that food spices are temporarily allowed to be used. Mainly used for preparing spices and mustard-type flavors for pickles, cans, sauces, seasonings, etc.
2. Used to prepare food additives, medicines, pesticides, fungicides, etc.
3. Used as fumigant, military poison gas, etc. [22]

 微信扫一扫打赏
微信扫一扫打赏

