overview【1】
3-nitrobenzamide, also called m-nitrobenzamide, is an organic intermediate that can be prepared from 3-nitrobenzaldehyde or m-nitrobenzonitrile.
preparation【1-2】
1.
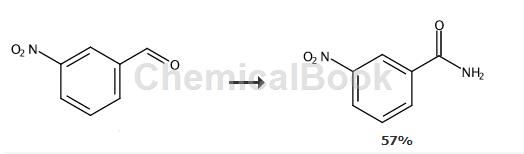
dissolve 100 mg of m-nitrobenzaldehyde, 56 mg of hydroxylamine hydrochloride, and 259 mg of cesium carbonate in a mixed solution of 1.5 ml of dimethyl sulfoxide and 0.5 ml of water. after stirring at 100°c for 7 hours, add 8 mg of palladium acetate. continue stirring for 12 hours. the reaction was monitored by tlc. after the reaction is completed, the reaction solution is cooled to room temperature, and an appropriate amount of water is added. extract with ethyl acetate, take the organic layer, dry it, filter it with suction, and evaporate the solvent under reduced pressure to obtain the crude product. after separation and purification by column chromatography, 63 mg of intermediate m-nitrobenzamide was obtained with a yield of 57%.
1h-nmr (400mhz, methanol-d4): δ8.78–8.72(m,1h),8.41(m,j=8.2,2.2,1.0hz,1h), 8.30–8.25(m,1h),7.74(t ,j=8.0hz,1h).
2.
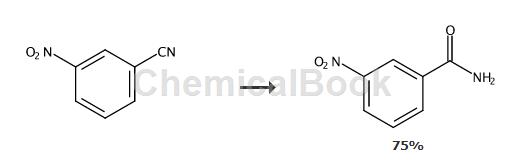
add csoh·h2o (0.0336g, 10mol%) and m-nitrobenzonitrile (2 mmol) in sequence to the reaction tube, then add ammonia water (1.0ml) as the solvent, seal the reaction tube and heat to 100°c for reaction 1h. the reaction conversion rate measured by gc‑ms was over 99%, and the product was separated and purified by column chromatography, with an isolation yield of 75%. 1h nmr (500mhz, d6‑dmso): δ8.69(d,j=2.0hz,1h),8.39(b,1h),8.36(d,j=8.0 hz,2h),8.31(d,j=7.5 hz,1h),7.77(m,1h),7.73(b,1h).13c nmr(125.4mhz, d6‑dmso):δ165.8,147.8,135.7,133.8,130.1,125.9,122.2.ms(ei):m /z(%)167 (6),166(67),151(8),150(100),105(3),104(33),103(8),92(17),77(14), 76(54), 75(32),74(32),73(6),65(26),64(6),63(9),62(5),53(4),52(5), 51(26),50(52), 46(20),44(50),39(9),38(8),37(5),30(31).
3.
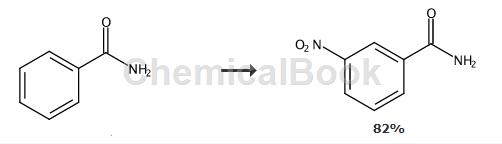
the general method is as follows: the synthesis of 3-nitrobenzamide refers to the synthesis of 4-nitrophenol below. to a solution of phenol (1 mmol) in dichloromethane were added 69% nitric acid (1 mmol) and 1 mmol transition metal complex ([co(nh3)5cl]cl2), and the reaction mixture was stirred at room temperature for 2 hours. the reaction progress was monitored by tlc. after indicating completion of the reaction by tlc, the reaction mixture was treated with sodium carbonate solution. the reaction mixture was extracted with dichloromethane. the organic layer was separated, dried over sodium sulfate and evaporated to give crude product. the crude product was purified on silica gel to give 4-nitrophenol as product. 3-nitrobenzamide, yield 82%.
references
【1】cn201410027159.2n, n-disubstituted benzazepine-2-amine compounds and their uses
【2】cn201210357102.x a method for preparing amides from nitriles【3】from synthetic communications, 41(19), 2946-2951; 2011

 微信扫一扫打赏
微信扫一扫打赏

