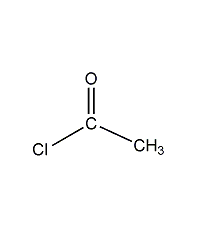
Structural formula
| Business number | 01JL |
|---|---|
| Molecular formula | C2H3ClO |
| Molecular weight | 78 |
| label |
Chloroacetyl, Acetyl chloride, Acetyl chloride, Ethanoyl chloride, Aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:75-36-5
MDL number:MFCD00000719
EINECS number:200-865-6
RTECS number:AO6390000
BRN number:605303
PubChem number:24845125
Physical property data
1. Characteristics: colorless fuming liquid with a strong pungent odor. [1]
2. Melting point (℃): -112[2]
3. Boiling point (℃): 51~52[3]
4. Relative density (water=1): 1.11 (20℃)[4]
5. Relative vapor density (air=1): 2.70[5]
6. Saturated vapor pressure (kPa): 32 (20℃)[6 ]
7. Heat of combustion (kJ/mol): -1099[7]
8. Critical temperature (℃): 246 [8]
9. Critical pressure (MPa): 5.83[9]
10. Octanol/water distribution Coefficient: -0.47[10]
11. Flash point (℃): 4 (CC)[11]
12. Ignition temperature (℃): 390[12]
13. Explosion upper limit (%): 19[13]
14. Lower explosion limit (%): 7.3[14]
15. Solubility: soluble in acetone, ether, acetic acid, benzene and chloroform. [15]
16. Solubility parameter (J·cm-3)0.5:20.189
17. van der Waals area (cm2·mol-1): 5.520×109
18. van der Waals volume (cm3·mol-1): 36.990
19. Liquid phase standard claims heat (enthalpy) (kJ· mol-1): -275.2
20. Liquid phase standard hot melt (J·mol-1·K-1): 115.0
21. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -242.8
22. Gas phase standard entropy (J·mol-1·K-1): 295.21
23. Gas phase standard free energy of formation (kJ·mol-1 ): -205.1
24. Gas phase standard hot melt (J·mol-1·K-1): 67.89 p>
Toxicological data
1. Acute toxicity[16] LD50: 910mg/kg (rat oral)
2. Irritation No data yet
3. Mutagenicity [17] Gene transformation and mitotic recombination: Drosophila melanogaster 62500μmol/L p>
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability[18] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3 When, the degradation half-life is 5a (theoretical).
4. Other harmful effects[19] This substance is dangerous to the environment.�, special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 16.19
2. Molar volume (cm3/mol): 70.0
3. Isotonic specific volume (90.2K ): 155.2
4. Surface tension (dyne/cm): 24.1
5. Polarizability (10-24cm3): 6.41
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 33
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It smokes in humid air and has a tear-inducing effect. Mixed with air can form explosive gas. If there is a spill or leak, neutralize it immediately with baking soda.
2. Stability[20] Stable
3. Incompatible substances[21] Water, alcohols, strong oxidants, strong alkali
4. Conditions to avoid contact [22] Heat, Moist air
5. Polymerization hazard[23] Does not polymerize
6. Decomposition products[ 24] Hydrogen chloride, phosgene
Storage method
Storage Precautions[25] Stored in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. The packaging must be sealed to prevent moisture. They should be stored separately from oxidants, alcohols, etc. and avoid mixed storage. It should not be stored for a long time to avoid deterioration. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Industrially, acetyl chloride can be produced by reacting ethylene with hydrogen chloride, or by reacting sodium acetate, sulfur dioxide and chlorine. Laboratory can be produced by reacting acetic acid, sodium acetate or acetic anhydride with various chlorinating agents. For example, it is obtained by reacting acetic anhydride with chlorosulfonic acid (or hydrogen chloride, carbon tetrachloride, or phosgene). It can also be obtained by reacting glacial acetic acid with benzoyl chloride (or silicon tetrachloride, phosphorus trichloride, chlorinated sulfurous acid, or phosphorus pentachloride). Operation Example 1: The ingredient ratio (molar ratio) is glacial acetic acid:phosphorus trichloride=3:1.2. Add glacial acetic acid to the reactor, stir slowly, and add phosphorus trichloride dropwise at room temperature within 10-15 minutes. Heating and raising the temperature, keeping the reaction at 40-50°C for 0.5 hours, leaving the reaction product standing, and then separation to obtain crude acetyl chloride, with a pure yield of about 70%. Operation Example 2 In a 3L three-necked flask, put 360g (6mol) glacial acetic acid and 552g toluene, heat to 50℃, add 510g (3mol) silicon tetrachloride dropwise within 30min, and keep it at 50℃ until the hydrogen chloride gas is no longer violent. until it escapes. Then carry out fractional distillation and steam until the temperature at the top of the column is 80-85°C to obtain the crude product. The fine product is distilled again, and 50-65 fractions are taken to obtain 400g of acetyl chloride with a yield of 85%. Raw material consumption quota: glacial acetic acid 850kg/t, phosphorus trichloride 1950kg/t.
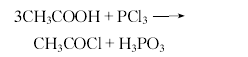
Purpose
1. Determination of cholesterol and moisture in organic liquids, lead hydroxytetraethyl, etc.
2. Used in the manufacture of organic compounds, dyes and pharmaceuticals. [26]

 微信扫一扫打赏
微信扫一扫打赏

