Background and overview[1][2]
Diphenhydramine citrate, usually diphenhydramine citrate, has antihistamine, sedative, local anesthesia, antiemetic and anti-M-cholinergic effects. It is commonly used clinically for skin and mucous membrane allergies, Acute allergic reactions, prevention and treatment of motion sickness, hypnosis and preoperative medication, dental local anesthesia, antitussive, etc. It can also be used to fight shaking paralysis, and can also be combined with other drugs to treat Parkinson’s disease and extrapyramidal symptoms. Compared with diphenhydramine hydrochloride, diphenhydramine citrate has enhanced stability, reduced bitterness, reduced irritation, increased fat solubility, and is easy to absorb.
Preparation method[1]
U.S. patents US4401665 and US4505862 disclose a method for synthesizing diphenhydramine citrate, which is obtained by reacting diphenhydramine hydrochloride with sodium hydroxide, and then reacting it with citric acid. Diphenhydramine Citrate. However, this method uses diphenhydramine hydrochloride as the starting raw material, and the cost is high.
U.S. patent US2421714 discloses two methods for synthesizing diphenhydramine: one is to react diphenylmethane with hydrogen bromide to prepare diphenyl bromide, and then the benzene-diphenyl bromide mixture is dissolved in anhydrous sodium carbonate React with dimethylaminoethanol in the presence of dimethylaminoethanol. Add water to the reactant to dissolve the salt and extract it with ether. The ether extract is extracted with hydrochloric acid solution. The hydrochloric acid extract is treated with sodium hydroxide to free diphenhydramine. After extraction with ether, it is evaporated. Dry the solvent, add benzene to dissolve it, evaporate the solvent to dryness, distill the residue under reduced pressure, and collect the 150~165°C/2mm fraction to obtain diphenhydramine; the second is to react dimethylaminoethanol and sodium in xylene to generate dimethyl Sodium aminoethoxide, remove excess sodium and then react with diphenylmethane bromide. The reaction solution is washed with water to remove salt and excess sodium dimethylaminoethoxide, and then distilled under reduced pressure to collect the 199~202℃/11mm fraction to obtain benzene. Lamin. However, the above two methods are cumbersome and complicated to operate, and the product yield is low.
CN201110116781.7 provides a synthesis method of diphenhydramine citrate, which has simple operation, high product yield, short production cycle, low cost, and is suitable for industrial production.
To achieve the above objectives, the present invention provides the following technical solutions:
The aqueous solution of benzyl alcohol-β-chloroethyl ether and excess dimethylamine is stirred and reacted at a temperature of 110~150℃ and a pressure of 0.5~2.5MPa (this reaction generates diphenhydramine hydrochloride, chemical reaction formula (as shown in formula I), after the reaction is completed, cool the reaction solution to 60~100°C, gradually remove the pressure during the cooling process, recover part of the unreacted dimethylamine gas, and then add an appropriate amount of NaOH aqueous solution under normal pressure conditions , stirring reaction (this reaction generates diphenhydramine, the chemical reaction formula is shown in Formula II; in addition to being a reaction substrate, NaOH also has the effect of reducing the solubility of dimethylamine in water), and heat it up to 100~150℃ (Because the reaction to generate diphenhydramine is very fast after adding NaOH, the stirring reaction and temperature rise can be carried out at the same time, that is, stirring and temperature rise directly after adding NaOH), recover the remaining unreacted dimethylamine gas, and finally react. The liquid is cooled to room temperature (room temperature in the present invention refers to 10~30°C), add citric acid solution, stir the reaction (this reaction generates diphenhydramine citrate, the chemical reaction formula is shown in formula III), filter, The filter cake is washed and dried to obtain diphenhydramine citrate.
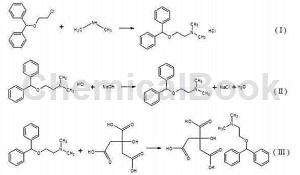
The beneficial effects of the present invention are: the present invention provides a “one-pot” method for synthesizing diphenhydramine citrate, which combines bisphenyl alcohol-β-chloroethyl ether React with dimethylamine to generate diphenhydramine hydrochloride, add NaOH to dissociate the diphenhydramine, and then directly add citric acid in the same reactor to perform an acid-base reaction to obtain diphenhydramine citrate. This method There is no need to separate and purify the intermediates diphenhydramine hydrochloride and diphenhydramine, which can greatly simplify the operation steps, reduce the loss of intermediates, increase product yield, shorten the production cycle, reduce production costs, and is suitable for industrial large-scale production. The starting material, benzyl alcohol-β-chloroethyl ether, can be obtained through commercial channels or prepared by oneself. In the present invention, benzyl alcohol and 2-chloroethanol are reacted in the presence of concentrated sulfuric acid to prepare benzyl alcohol-β-chloroethyl ether. Ether, without using toxic organic solvents, not only simplifies subsequent purification steps, but also improves personnel operation and product safety.sex. Combining the above-mentioned synthesis method of benzyl alcohol-β-chloroethyl ether and the synthesis method of diphenhydramine citrate has significant effects in simplifying operating steps, increasing product yield, and reducing production costs.
Preparations[2]
CN201110432644.4 discloses a diphenhydramine citrate orally disintegrating tablet. The prescription consists of the following components in terms of mass percentage: diphenhydramine citrate 19%, filler 45~58%, Disintegrant 15~25%, effervescent disintegrant 4~8%, lubricant 0.5~1%, glidant 1~3%, sweetener 1~2%, aromatic agent 0.2~0.6% and surface activity 0~0.5%; the filler is mannitol or a combination of mannitol and lactose or erythritol; the disintegrant is crospovidone, croscarmellose sodium and low-substituted hydroxypropyl cellulose Any of them are combined with microcrystalline cellulose. The effervescent disintegrant is citric acid and sodium bicarbonate with a mass ratio of 1 to 2:1, the lubricant is magnesium stearate, and the glidant is micronized silica gel or talc. Powder, the sweetener is aspartame or stevia, the aromatic agent is pharmaceutically acceptable essence, and the surfactant is sodium lauryl sulfate; the preparation method of the orally disintegrating tablet is also disclosed, which is easy to operate. The cost is low, and the obtained product meets the quality requirements of orally disintegrating tablets, with beautiful appearance, good taste and stable quality.
Main reference materials
[1] CN201110116781.7 Synthesis method of diphenhydramine citrate
[2] CN201110432644.4 Diphenhydramine citrate orally disintegrating tablets and preparation method thereof

 微信扫一扫打赏
微信扫一扫打赏

