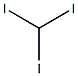
Structural formula
| Business number | 01JM |
|---|---|
| Molecular formula | CHI3 |
| Molecular weight | 393.73 |
| label |
Seaiodoform, yellow iodine, iodoform, IPG Dry Adhesive Strips, Carbon triiodide, Triiodomethane |
Numbering system
CAS number:75-47-8
MDL number:MFCD00001069
EINECS number:200-874-5
RTECS number:PB7000000
BRN number:1697010
PubChem number:24881012
Physical property data
1. Characteristics: yellow powder or crystal with unpleasant odor. [1]
2. Melting point (℃): 115~120[2]
3. Boiling point (℃) :218[3]
4. Relative density (water = 1): 4.01[4]
5. Relative Vapor density (air = 1): 13.0[5]
6. Octanol/water partition coefficient: 3.03[6]
7. Solubility: Slightly soluble in water, soluble in benzene, ether and acetone. [7]
8. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): 141.0
9. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): 210.9
10. Gas phase standard entropy (J·mol-1 ·K-1): 355.62
11. Gas phase standard free energy of formation (kJ·mol-1): 178.0
12. Vapor phase standard hot melt (J·mol-1·K-1): 75.07
Toxicological data
1. Acute toxicity[8]
LD50: 355mg/kg (rat oral); 1184mg/kg (rabbit dermal )
LC50: 2657mg/m3 (mouse inhalation, 7h)
2. Irritation No information available
3. Mutagenicity [9] Microbial mutagenicity: Salmonella typhimurium 67μg/dish. Unprogrammed DNA synthesis and sister chromosome exchange in hamster embryos: 1mg/L.
Ecological data
1. Ecotoxicity[10] LC50: 2.92mg/L (96h) (fathead minnow, dynamic)
2. Biodegradability No data yet
3. Non-biodegradability [11] In the air, when the concentration of hydroxyl radicals When the concentration is 5.00×105 pieces/cm3, the degradation half-life is 55d (theoretical).
4. Other harmful effects [12] This substance is harmful to the environment and attention should be paid to atmospheric pollution.
Molecular structure data
1. Molar refractive index: 45.54
2. Molar volume (cm3/mol): 101.8
3. Isotonic specific volume (90.2K ): 291.2
4. Surface tension (dyne/cm): 66.7
5. Polarizability (10-24cm3): 18.05
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.7
2. Number of hydrogen bond donors: 0
3. Hydrogen bond numberNumber of isomers: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: None
6. Topological molecular poles Surface area 0
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 8
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13 .Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[13] Stable
2. Incompatible substances[14] Strong oxidants, strong bases, alkali metals, mercury and its compounds
3. Conditions to avoid contact [15] Light, heat , friction, impact
4. Polymerization hazard[16] No polymerization
5. Decomposition products[17] Hydrogen iodide
Storage method
Storage Precautions[18] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Avoid light. The packaging is sealed. They should be stored separately from oxidants, alkalis, alkali metals, and food chemicals, and avoid mixed storage. Suitable materials should be available in the storage area to contain spills.
Synthesis method
It is obtained by halogenation and hydrolysis of acetone (or ethanol). First add water, sodium iodide and acetone into the reaction pot, add ice and cool down to 10°C. Slowly add sodium hypochlorite while stirring until the end point is reached when no turbidity occurs, and control the temperature not to exceed 20°C. Let it stand for 1 hour, suck off the supernatant, take out the iodoform layer and filter. Wash the filter cake with water until it is neutral, and then wash it with distilled water until there is no chlorine radical. Then dry it at 35-40℃ to get the finished product. In addition, this product can also be prepared by reacting chloroform with methyl iodide.
Purpose
1. Used as a preservative and disinfectant in medicine and biochemistry.
2. Used as chemical intermediates and preservatives. [19]

 微信扫一扫打赏
微信扫一扫打赏

