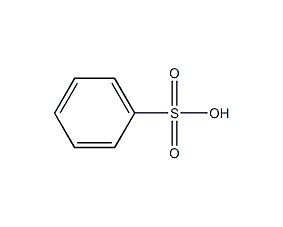
Structural formula
| Business number | 02DC |
|---|---|
| Molecular formula | C6H6O3S |
| Molecular weight | 158.18 |
| label |
aromatic sulfur compounds |
Numbering system
CAS number:98-11-3
MDL number:MFCD00011689
EINECS number:202-638-7
RTECS number:DB4200000
BRN number:742513
PubChem number:24847719
Physical property data
1. Properties: Colorless needle-like or flaky crystals.
2. Density (g/mL, 25℃): 1.32
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 44
5. Boiling point (ºC, normal pressure): 137
6. Boiling point (ºC, kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 20ºC): Not determined
12. Saturated vapor pressure (kPa, 120ºC ): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical Pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water, easily soluble in ethanol, slightly soluble in benzene, Insoluble in ether and carbon disulfide.
Toxicological data
1. Skin/eye irritation: Start irritation test: rabbit skin contact, 100μg/24H; Standard Dresser test: rabbit skin contact, 2mg/24HREACTION SEVERITY, strong reaction; Standard Dresser test: rabbit eye contact, 250μg/ 24HREACTION SEVERITY, strong reaction; 2. Acute toxicity: Rat oral LD50: 890μL/kg; Cat skin contact LDL0: 10mg/kg; Wild birds oral LD50: 75mg/kg;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 37.10
2. Molar volume (cm3/mol): 112.2
3. Isotonic specific volume (90.2K ): 300.2
4. Surface tension (dyne/cm): 51.2
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Pole Transformation rate: 14.70
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 0
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 54.4
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 184
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidants, strong acids, and alkalis.
Irritating to skin, eyes and respiratory tract. Its sodium salt is non-toxic. Protective equipment should be worn during operation. Rat oral LD50890mg/kg, cat transdermal LD5010mg/kg.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, acids, alkalis, and food chemicals, and avoid mixed storage. Suitable materials should be available in the storage area to contain spills.
This product is mostly for personal use and is rarely sold. Generally packed in plastic barrels, tightly sealed, and stored in a sun-proof, moisture-proof, and well-ventilated place
Synthesis method
Using benzene as raw material, it is obtained by sulfonation with sulfuric acid. Add 2400L of 93% sulfuric acid into the sulfonation pot, and pump benzene into the evaporator at a flow rate of 2500-3000L/h. After evaporation and superheating, it becomes superheated benzene vapor with a temperature above 150°C, and is carried out in the sulfuric acid layer through the bubbler. The reaction temperature is around 170°C, and the final free acid is controlled at 4-5%. The reaction is completed in about 10 hours. The unreacted benzene vapor and the generated water vapor are separated by condensation. The acidic benzene is neutralized with liquid alkali and dehydrated with salt before recycling. The benzene-containing benzene sulfonic acid is then passed through a vacuum benzene removal device to remove residual benzene. The sulfonation yield is 96.5%. Neutralize benzenesulfonic acid with sodium sulfite or sodium hydroxide to obtain sodium benzenesulfonate. Neutralization yield 99%. Raw material consumption quota: benzene 600kg/t, oleum 750kg/t.
![]()
Purpose
Mainly used to make phenol through alkali melting, also used to make resorcinol, etc., and also used as a catalyst.

 微信扫一扫打赏
微信扫一扫打赏

