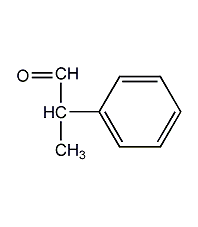
Structural formula
| Business number | 026E |
|---|---|
| Molecular formula | C9H10O |
| Molecular weight | 134.18 |
| label |
Hydrogenated cinnamic aldehyde; solanaldehyde, Hydrogenated cinnamaldehyde, Black nightshade aldehyde, artificial flavors |
Numbering system
CAS number:93-53-8
MDL number:MFCD00006973
EINECS number:202-255-5
RTECS number:CY1460000
BRN number:4291321
PubChem number:24878254
Physical property data
1. Properties: water white to light yellow liquid. It has a scent similar to narcissus, grass leaves and melon.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): Undetermined
5. Boiling point (ºC, normal pressure): 222
6. Boiling point (ºC, 2kPa):
7. Refractive index: Undetermined
8. Flash point (ºC): 69
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V) : Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Lml dissolved in 1.5mL 70% ethanol, soluble in most non-toxic Volatile oil and mineral oil, insoluble in glycerol, slightly soluble in propylene glycol.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 40.62
2. Molar volume (cm3/mol): 136.8
3. Isotonic specific volume (90.2K): 331.1
4. Surface tension (dyne/cm): 34.3
5. Polarizability (10-24cm3): 16.10
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.9
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 2
6.Topological molecule polar surface area 17.1
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 103
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 1
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
Water white to light yellow liquid. It has a scent similar to narcissus, grass leaves and melon. Soluble in most non-volatile oils and mineral oils, insoluble in glycerin, slightly soluble in propylene glycol.
Storage method
This product should be sealed and stored in a cool place.
Synthesis method
1. It is formed by the condensation of acetophenone and ethyl chloroacetate under alkaline conditions.
2. Obtained from methylphenyl glycol distilled under normal pressure.
3. Preparation method:
Ethyl 3-phenyl-2,3-epoxybutyrate (4): in a reaction bottle equipped with a stirrer and thermometer, Add 60g (0.5mol) of acetylbenzene (2), 61.5g of ethyl chloroacetate (3), and 100mL of benzene dried with metallic sodium. Cool in an ice-water bath, add 23.6g of sodium amide with stirring for 2 hours. The organic layer was separated, and the aqueous layer was extracted with 100 mL benzene. Combine the organic layers, wash twice with water, and then wash with 100 mL of water containing 5 mL of acetic acid. Dry over anhydrous magnesium sulfate, remove benzene by distillation, and fractionate under reduced pressure. Collect the fraction at 111~114℃/0.4kPa to obtain 67g of ethyl 3-phenyl-2,3-epoxybutyrate (4), with a yield of 65%. . 2-Phenylpropionaldehyde (1): In a reaction bottle equipped with a stirrer and thermometer, add 150 mL of absolute ethanol, and add 7.6 g (0.33 mol) of metallic sodium in batches to prepare an ethanol solution of sodium ethoxide. Slowly add 66.5g of compound (4), cool to 15°C, and then slowly add 6 mL of water. The reaction is exothermic and the sodium salt is quickly precipitated. The mixture was allowed to react overnight, and the excess sodium salt that precipitated was washed with a small amount of ethanol and then with diethyl ether. Add the salt to dilute hydrochloric acid (prepared from 28 mL concentrated hydrochloric acid and 150 mL water), and heat the reaction in a steam bath for 1.5 h. Carbon dioxide escapes and oily substance precipitates. Cool and extract with benzene. Wash with water, steam out benzene, and then distill under reduced pressure. Collect the fraction at 90~93℃/1.33kpa to obtain 30g of 2-phenylpropionaldehyde (1), with a yield of 70%. [1]
Purpose
GB 2760–1996 stipulates the edible spices allowed to be used. Mainly used to prepare melon, almond, cherry, peach, plum and other types of flavors.

 微信扫一扫打赏
微信扫一扫打赏

