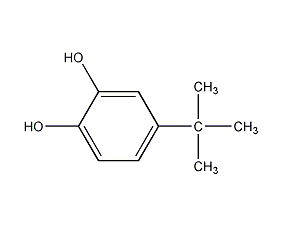
Structural formula
| Business number | 02DP |
|---|---|
| Molecular formula | C10H14O2 |
| Molecular weight | 166.22 |
| label |
p-tert-butylcatechol, 4-tert-butyl-1,2-benzenediol, p-tert-butylcatechol, 4-(1,1-dimethylethyl)-1,2-benzenediol, 4-bert-Butyl-1,2-benzenediol, 4-Tert-butyl-pyrocatechol, 4-(1,1-Dimethylethyl)-1,2-benzenediol, 1,2-Dihydroxy-4-tert-butylbenzene, p-tert-Butylcatechol, Highly efficient polymerization inhibitor, Antioxidants |
Numbering system
CAS number:98-29-3
MDL number:MFCD00002201
EINECS number:202-653-9
RTECS number:UX1400000
BRN number:2043335
PubChem number:24851787
Physical property data
1. Character: Colorless needle-like crystal
2. Relative density (60℃/25℃): 1.0490
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 52~55
5. Boiling point (ºC, normal pressure): 285
6. Boiling point (ºC, 99.4kPa): Undetermined
7. Refractive index: 1.508
8. Flash Point (ºC): 151
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 40ºC): Undetermined
12. Saturation vapor pressure (kPa, 20ºC): Undetermined
13. Heat of combustion (KJ/mol) : Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol /water) logarithmic value of the distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V) : Undetermined
19. Solubility: Slightly soluble in hot water, soluble in ethanol, ether, acetone, etc., insoluble in water and petroleum ether.
Toxicological data
1. Skin/eye irritation: Standard Dresser test: rabbit skin contact, 750μg/24HREACTION SEVERITY, strong reaction; 2. Acute toxicity: rat oral LD50: 2820mg/kg; rat skin contact LDL0: 2mg/kg ; Mice intravenously injected LD50: 32mg/kg; Rabbit skin contact LD50: 630μL/kg; 3. Mutagenicity: Mutation experiment in mammalian somatic cells: mouse lymphocytes, 80μg/L;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 48.40
2. Molar volume (cm3/mol): 152.9
3. Isotonic specific volume (90.2K ): 385.3
4. Surface tension (dyne/cm): 40.2
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 19.18
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 10
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 148
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid light and contact with strong oxidants, strong acids, and acid anhydrides.
2. Exist in smoke.
3. Toxic!
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, acids, etc., and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
(1) Obtained from the reaction of catechol and tert-butanol. Put xylene, catechol and phosphoric acid into the reaction kettle in sequence, stir and heat to dissolve, continue to heat until xylene refluxes, then slowly add tert-butyl dropwise Alcohol xylene solution, continue stirring for 2 hours after adding, and cool down. The material is then sent to a neutralization washing tank to stand, separated to remove phosphoric acid, filtered and recycled; the xylene layer is neutralized with sodium carbonate to a pH value of 5-6, washed with water, and then distilled under reduced pressure to obtain the finished product. When purification is required, petroleum ether recrystallization can be used. 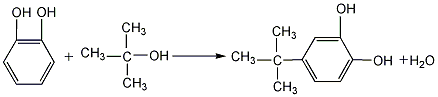 (2) Phosphoric acid, xylene, and phthalate are obtained from the reaction of catechol and isobutylene. Add the diphenol into the reaction kettle, stir and mix, and then heat to 80-90°C. Then continue to raise the temperature to 105~110°C and introduce isobutylene. After passing through enough isobutylene, cool it to 50~60℃ and let it stand for 5~6 hours. Dilute the emulsion with an equal volume of water, centrifuge and filter, and wash the filtrate with saturated sodium chloride solution until the pH value is 6. Recover xylene by heating and distillation, and then distill under reduced pressure to collect the 170-173°C (1.33kPa) fraction, which is the finished product.
(2) Phosphoric acid, xylene, and phthalate are obtained from the reaction of catechol and isobutylene. Add the diphenol into the reaction kettle, stir and mix, and then heat to 80-90°C. Then continue to raise the temperature to 105~110°C and introduce isobutylene. After passing through enough isobutylene, cool it to 50~60℃ and let it stand for 5~6 hours. Dilute the emulsion with an equal volume of water, centrifuge and filter, and wash the filtrate with saturated sodium chloride solution until the pH value is 6. Recover xylene by heating and distillation, and then distill under reduced pressure to collect the 170-173°C (1.33kPa) fraction, which is the finished product. 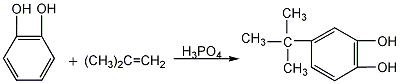 3.By catechol and The reaction of tert-butyl alcohol yields xylene, catechol and phosphoric acid. Stir and heat to dissolve. Continue to raise the temperature until xylene refluxes. Add the xylene solution of tert-butanol dropwise to carry out the reaction. After cooling down and letting it stand, the xylene layer is neutralized with sodium carbonate, washed with water, and then distilled under reduced pressure to obtain the finished product. Or it can be obtained by the reaction between catechol and isobutylene. Add phosphoric acid, xylene and catechol into the reaction kettle, raise the temperature to a certain temperature and pass in the isobutylene. After the reaction is completed, centrifuge the filtrate and wash the filtrate with saturated sodium chloride solution until the pH value is 6. Distillate to recover xylene, distill under reduced pressure and collect.
3.By catechol and The reaction of tert-butyl alcohol yields xylene, catechol and phosphoric acid. Stir and heat to dissolve. Continue to raise the temperature until xylene refluxes. Add the xylene solution of tert-butanol dropwise to carry out the reaction. After cooling down and letting it stand, the xylene layer is neutralized with sodium carbonate, washed with water, and then distilled under reduced pressure to obtain the finished product. Or it can be obtained by the reaction between catechol and isobutylene. Add phosphoric acid, xylene and catechol into the reaction kettle, raise the temperature to a certain temperature and pass in the isobutylene. After the reaction is completed, centrifuge the filtrate and wash the filtrate with saturated sodium chloride solution until the pH value is 6. Distillate to recover xylene, distill under reduced pressure and collect.
Purpose
1. Used as polymerization inhibitor and antioxidant. 2. The polymerization inhibitory effect is 25 times higher than that of hydroquinone at 60°C. It is a highly efficient polymerization inhibitor during the distillation, storage and transportation of olefin monomers. It is commonly used in styrene, butadiene, chloroprene, and isoprene. Alkene and other monomers are also used in vinyl chloride, vinyl pyridine, α-olefins, nonene, cyclopentadiene, acrylic acid, methacrylic acid and their esters, chlorinated olefins, polyurethane, etc. This product is also used as an antioxidant for polyethylene, polypropylene, polychloroprene, synthetic rubber, nylon and other polymers, as well as for grease and its derivatives, ethyl cellulose, caprolactam, maleic anhydride, lubrication Antioxidant for various compounds such as oil and metal soaps such as tin.
3.The polymerization inhibitory effect of p-tert-butylcatechol at 60℃ is 25 times higher than that of hydroquinone, which is the best value when distilling, storing and transporting olefin monomers. High-efficiency polymerization inhibitor, commonly used in monomers such as styrene, butadiene, chloroprene, isoprene, etc., and also used in vinyl chloride, vinyl pyridine, olefins, nonene, cyclopentadiene, acrylic acid, Methacrylic acid and its esters, chlorinated olefins, polyurethane, etc. It is also used as an antioxidant for polyethylene, polypropylene, polychloroprene, synthetic rubber, nylon and other polymers, as well as for grease and its derivatives, ethyl cellulose, caprolactam, maleic anhydride, lubricants and Antioxidants for various compounds such as tin and other metal soaps. 4.Used as an efficient polymerization inhibitor for styrene, butadiene and other olefin monomers. During the preparation, storage and transportation of polymer monomers, in order to prevent the monomers from self-polymerization or the formation of terminal polymers, certain TBC products need to be added to achieve the desired effect. Polymerization inhibition.TBC products have a special hindered phenol structure and are effectively used in polyethylene, polypropylene, synthetic rubber, nylon and other polymers; lubricants, caprolactam, horse Used as antioxidant in products such as anhydride. It is also used as an intermediate for fine chemicals such as medicines, pesticides, dyes, and spices; a stabilizer for organic epoxides and alcohol compounds; and a passivator for urethane catalysts.
style=”font-family:Arial;”>Used as an efficient polymerization inhibitor for styrene, butadiene and other olefin monomers. During the preparation, storage and transportation of polymer monomers, in order to prevent the monomers from self-polymerization or the formation of terminal polymers, certain TBC products need to be added to achieve the desired effect. Polymerization inhibition.TBC products have a special hindered phenol structure and are effectively used in polyethylene, polypropylene, synthetic rubber, nylon and other polymers; lubricants, caprolactam, horse Used as antioxidant in products such as anhydride. It is also used as an intermediate for fine chemicals such as medicines, pesticides, dyes, and spices; a stabilizer for organic epoxides and alcohol compounds; and a passivator for urethane catalysts.

 微信扫一扫打赏
微信扫一扫打赏

