
Structural formula
| Business number | 02DW |
|---|---|
| Molecular formula | C7H4F3NO2 |
| Molecular weight | 191 |
| label |
Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:98-46-4
MDL number:MFCD00007260
EINECS number:202-670-1
RTECS number:XT3500000
BRN number:2051362
PubChem number:24849283
Physical property data
1. Properties: colorless liquid.
2. Density (g/mL, 25℃): 1.436
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): -5
5. Boiling point (ºC, normal pressure): 200-205
6. Boiling point (ºC, kPa): Undetermined
p>
7. Refractive index: 1.472
8. Flash point (ºC): 87
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): 475
11. Vapor pressure (mmHg, 40ºC): Undetermined
12. Saturated vapor pressure (kPa, 20ºC ): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical Pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V): 18.6
18. Lower explosion limit (%, V/V): 1.2
19. Solubility: soluble in ethanol, ether, benzene, toluene and other solvents.
Toxicological data
Acute toxicity: Rat oral LD50: 610mg/kg; Rat inhalation LC50: 870 mg/kg; Mouse oral LD50: 520mg/kg; Mouse inhalation LC50: 880 mg/m3/2H; Mouse peritoneal cavity LD50 :100mg/kg; Amphibian-frog subcutaneous LDL0: 573mg/kg;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 37.77
2. Molar volume (cm3/mol): 134.7
3. Isotonic specific volume (90.2K ): 319.9
4. Surface tension (dyne/cm): 31.7
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 14.97
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 45.8
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 199
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidizing agents.
This product is toxic. Mildly irritating to skin and mucous membranes. Decomposition by heat releases toxic fumes of nitrogen oxides and fluoride.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills. Packed in 250kg plastic-lined iron drum. Store in a cool, ventilated warehouse. Store and transport separately from edible raw materials and oxidants.
Synthesis method
1. Obtained from nitration of benzene trifluoride. Add concentrated sulfuric acid into the reaction pot, add fuming sulfuric acid and concentrated nitric acid while stirring and cooling, heat to 40°C, add trichlorotoluene dropwise, and control the temperature to 40-45°C. After the addition is completed, react at 50-55°C for 3 hours, cool to room temperature, and let stand to separate the lower acid solution. Put the upper liquid into 5 times the amount of ice water, let it stand for layering, and wash the separated nitro compounds with 2% sodium carbonate solution and water to obtain m-nitrotrifluorotoluene. The yield is close to 93%.
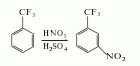
2. The preparation method is: Add concentrated sulfuric acid and concentrated nitric acid to the reaction bottle, start stirring, add trifluorotoluene dropwise, and control the reaction temperature at about 50°C. After the dropwise addition, continue stirring for 2 hours, then leave to separate and separate the waste acid and organic matter. The phase is washed with water, alkali and water in sequence until pH=7~8. The crude product contains approximately 91% meta-body, 6.8% ortho-body and 2.0% para-body. The crude product is fractionated to obtain 3-trifluoromethyl. Nitrobenzene.
Purpose
Used as pharmaceutical, pesticide, and dye intermediates.

 微信扫一扫打赏
微信扫一扫打赏

