
Structural formula
| Business number | 01KQ |
|---|---|
| Molecular formula | C2HF3O2 |
| Molecular weight | 114 |
| label |
perfluoroacetic acid, trifluoroacetic acid, Trifluoroacetic acid, Trifluoroacetic acid (TFA), perfluoroacetic acid, Trifluoracetic acid, Aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:76-05-1
MDL number:MFCD00004169
EINECS number:200-929-3
RTECS number:AJ9625000
BRN number:742035
PubChem number:24900380
Physical property data
1. Characteristics: Colorless, transparent, hygroscopic fuming liquid with a strong pungent odor. [11]
2. Melting point (℃): -15.2[12]
3. Boiling point (℃): 72.4~74[13]
4. Relative density (water = 1): 1.54[14]
5. Relative vapor density (air = 1): 3.9[15]
6. Saturated vapor pressure (kPa): 14.23 (25℃)[16]
7. Critical pressure (MPa): 3.26[17]
8. Octanol/water partition coefficient: 0.5[18]
9. Solubility: Easily soluble in water, ethanol, ether, acetone, and benzene. [19]
10. Viscosity (mPa·s, 20ºC): 0.926
11. Relative density (25℃, 4℃): 1.486
12. Relative density (20℃, 4℃): 1.53510
13. Solubility parameter (J·cm-3)0.5: 21.621
14. van der Waals area (cm2·mol-1): 6.510 ×109
15. van der Waals volume (cm3·mol-1): 41.070
16. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -1031.4
17. The liquid phase standard claims heat (enthalpy) (kJ· mol-1): -1069.9
18. Liquid phase standard hot melt (J·mol-1·K-1): 170.2
Toxicological data
1. Acute toxicity: Rat oral LD50: 500mg/kg; rat inhalation LC50: 10mg/m3; mouse inhalation LC50: 13500mg/m3; Mouse intraperitoneal LDLo: 150mg/kg; Mouse intravenous LD50: 1200mg/kg;
2. It is moderately toxic. It has an irritating effect on the skin, eyes and mucous membranes. Its irritation is stronger than trichloroacetic acid, and its penetration into tissues is more obvious. Causes severe pain and burns on contact with skin. When the skin of guinea pigs or rats comes into contact with a trifluoroacetic acid solution of 20% or higher concentration, it causes obvious coagulation necrosis or complete tissue dissolution. The maximum allowable concentration in the workplace is 0.025~0.05mg/L.
3. Acute toxicity [20]
LD50: 200mg/kg (rat oral)
LC50: 1000mg/m3 (rat inhalation)
Ecological data
Slightly hazardous to water, avoid contact of undiluted or large quantities of product with groundwater, waterways or sewage systems. Harmful to fish.
Molecular structure data
The � group is a group with good lipophilicity and strong electronegativity. It plays a special role in biologically active molecules. Trifluoromethyl group (Formula 2, Formula 3) [6,7] can be introduced into some compound molecules using trifluoroacetic acid.
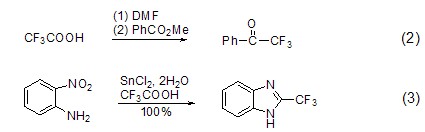
Carboxylate ester SynthesisTrifluoroacetic anhydride reacts with hydrogen peroxide to generate peroxytrifluoroacetic acid, which can be used to cause the Baeyer-Villiger oxidation reaction of some ketones to generate the corresponding carboxylic acid ester (Formula 4)[8] .
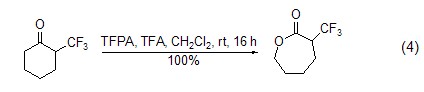
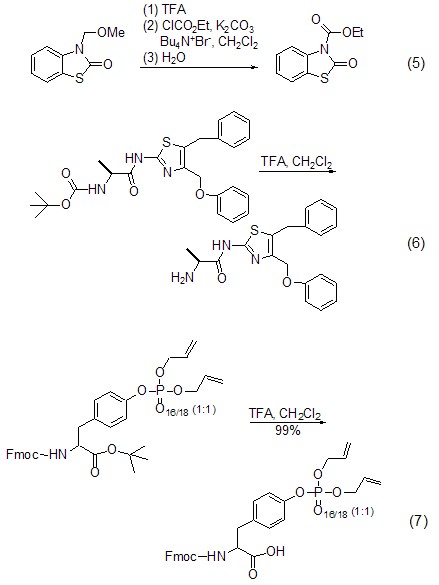
Used for deprotection ReagentsIn organic reactions, in order to selectively carry out a certain reaction while retaining other more active chemical groups (such as amino groups, hydroxyl groups, etc.), it is often necessary to use some protective groups to protect them. Trifluoroacetic acid can easily remove the protective groups of amino and hydroxyl groups [5], for example, N-benzyloxycarbonyl, N -Methoxymethyl (formula 5), N-tert-butoxycarbonyl (formula 6)[9], O-tert-butoxy Carbonyl (formula 7)[10], N-benzyloxymethyl, etc.
4. Used as test reagents, Solvents, catalysts and used in organic synthesis. [27]

 微信扫一扫打赏
微信扫一扫打赏

