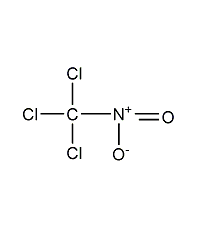
Structural formula
| Business number | 01KR |
|---|---|
| Molecular formula | CCl3NO2 |
| Molecular weight | 164.38 |
| label |
Trichloronitromethane, Nitrochloroform, nitrochloroform, Nitrochloroform, Trichloromethane, Chloropicrin, Nitrotrichloromethane, Aquinite, Trichloronitromethane, pesticides |
Numbering system
CAS number:76-06-2
MDL number:MFCD00041878
EINECS number:200-930-9
RTECS number:PB6300000
BRN number:1756135
PubChem number:24867884
Physical property data
1. Properties: colorless or slightly yellow oily liquid with tear-inducing properties. [1]
2. Melting point (℃): -69.2[2]
3. Boiling point (℃): 112[3]
4. Relative density (water=1): 1.66 (20℃)[4]
5. Relative vapor density (air=1): 5.7[5]
6. Saturated vapor pressure (kPa): 2.26 (20℃)[6]
7. Octanol/water partition coefficient: 2.09[7]
8. Solubility: insoluble in water, soluble in ethanol, Most organic solvents such as benzene and carbon disulfide. [8]
Toxicological data
1. Acute toxicity[9] LD50: 126~271mg/kg (mouse oral)
2. Irritation Properties No data available
3. Mutagenicity [10] Microbial mutagenicity: Salmonella typhimurium 50μg/dish, large intestine Bacillus 50μg/dish. Sister chromatid exchange: human lymphocytes 8mg/L.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 27.21
2. Molar volume (cm3/mol): 92.0
3. Isotonic specific volume (90.2K ): 240.9
4. Surface tension (dyne/cm): 46.9
5. Polarizability (10-24cm3): 10.78
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.1
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 43.1
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 79.9
10. Number of isotope atoms: 0
11. The number of determined atomic stereocenters: 0
12. The number of uncertain atomic stereocenters: 0
13, The number of determined stereocenters of chemical bonds: 0
14, The number of uncertain stereocenters of chemical bonds: 0
15, The number of covalent bond units: 1
Properties and stability
1. Stability[11] Stable
2. Incompatible substances[12] Strong reducing agents, inorganic bases, alkali metals, halogenated alkanes, metal hydrides, metal alkoxides, ammonia, amines, fuming sulfuric acid, etc.
3. Conditions to avoid contact [13] Heating
4. Polymerization hazard[14] No polymerization
5. Decomposition products[15] Chloride, nitrogen oxides, carbon
Storage method
Storage Precautions[16] Store in a cool, well-ventilated special warehouse, and implement the “two people to send and receive, and two people to keep” system. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents, acids, and food chemicals, and avoid mixed storage. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Main raw materials: trinitrophenol, calcium hydroxide, chlorine. Add lime to water in a certain proportion to prepare lime slurry, then mix a certain amount of lime, trinitrophenol, and water to make calcium salt and send it to the chlorination kettle, add lime slurry, and pass chlorine for about 2 hours under freezing. The temperature is controlled at 32±1°C, and then cooled to 7±1°C. After chlorination, it is distilled.
Purpose
1. This product is an alert fumigant that can kill insects, bacteria, and mice. It can also be used for fumigation of food pests. It can also be used for wood preservation, house floors, ship disinfection, soil, and plants. Seed disinfection, etc.
2. Used in organic synthesis to manufacture dyes, pesticides, fungicides, etc. [17]

 微信扫一扫打赏
微信扫一扫打赏

