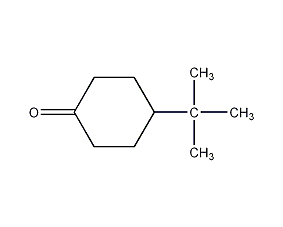
Structural formula
| Business number | 02E1 |
|---|---|
| Molecular formula | C10H18O |
| Molecular weight | 154.25 |
| label |
p-tert-butylcyclohexanone, Tert-butyl cyclohexanone, artificial flavors |
Numbering system
CAS number:98-53-3
MDL number:MFCD00001642
EINECS number:202-678-5
RTECS number:GW1140000
BRN number:507309
PubChem number:24851872
Physical property data
1. Appearance: white crystal
2. Density (g/mL, 25℃): 0.893
3. Melting point (ºC): 47-50
4. Boiling point (ºC, 20mmHg): 113-116
5. Refractive index: 1.447
6. Flash point (ºC): 96
7. Autoignition point or ignition temperature (ºC): 440
Toxicological data
1. Acute toxicity: rat oral LD50: 5mg/kg; rabbit skin contact LD50: 5mg/kg.
2. No irritation was found after applying the product on rabbit skin for 1 day under closed conditions. If a Vaseline preparation with a concentration of 6% is used in a closed skin contact test on humans, it will not cause irritation. Similarly, the highest limit of testing on humans did not produce allergic reactions.
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 45.99
2. Molar volume (cm3/mol): 169.1
3. Isotonic specific volume (90.2K): 400.3
4. Surface tension (dyne/cm): 31.3
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability: 18.23
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 2
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 143
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidizing agents.
White crystalline solid. It has a woody, mint-like aroma with a hint of patchouli.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation�Facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Use phenol as the starting material to undergo Friedel-Crafts reaction for tert-butylation, then catalytic hydrogenation to generate the corresponding tert-butylcyclohexanol, and then oxidize it with chromium trichloride to convert it into this product.
2. Preparation method:

Into a reaction bottle equipped with a stirrer, thermometer, and ventilation tube, add 200 mL of toluene and 8 g of NCS (0.06 mol), add nitrogen, and cool to 0°C while stirring. Add 6 mL (0.1 mol) of dimethyl sulfide, cool to -25°C, and add dropwise a solution of 4-tert-butylcyclohexanol (2) and 40 mL of toluene for about 5 minutes. After the addition was completed, the reaction was stirred at -25°C for 2 hours. Add dropwise a solution of 6g (0.06mol) triethylamine and 10mL toluene, and complete the addition in about 3 minutes. Remove the cold bath, add 400 ml of diethyl ether after 5 minutes, wash with 100 mL of 1% dilute hydrochloric acid and 100 mL of water x 2, dry over anhydrous sodium sulfate, and concentrate under reduced pressure. The residue was distilled under reduced pressure, and the fraction at 120°C/3.325kPa was collected to obtain the product 4-tert-butylcyclohexanone ① (1) 5.54-5.72g, with a yield of 90%-93%. Note: ① This is a method to oxidize primary and secondary alcohols into carbonyl compounds with high yield. The following various carbonyl compounds can be synthesized using this method (Table I=7-5 page 286). [1]
3. Preparation method:
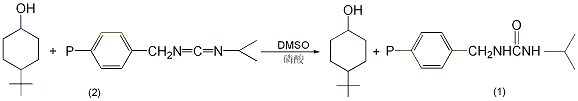
In a reaction flask equipped with a stirrer and ventilation tube , add 540 mg (3.46 mmol) 4-tert-butylcyclohexanol (2), 50 mL anhydrous benzene, and 0.2 mL anhydrous dimethyl sulfoxide containing 98 mg (1 mmol) anhydrous phosphoric acid ①. Pour in nitrogen, add 13.19g of polymer-supported carbodiimide resin (containing 0.012 mol of activated carbon diimide) with stirring, and stir for 3.5 days at room temperature. Filter and wash with ether three times, 100 mL each time. Combine the organic layers and wash with water 5 times, 100 mL each time. Concentrate to dryness to obtain 446-450 mg of product (1), with a yield of 83%-84%. mp42~45℃. Note: ① Anhydrous phosphoric acid is prepared as follows: add 3.98g of phosphorus pentoxide to 5.58mL of 85% phosphoric acid, and heat for 15 minutes to completely dissolve; or add 27mL of water dropwise to 71g of phosphorus pentoxide while cooling in an ice bath. Just dissolve it completely. [2]
4. Preparation method:
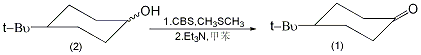
Equipped with stirrer, thermometer and dropping funnel , add 8.0g of CBS (0.06mol) and 200mL of toluene into the reaction bottle of the ventilation tube, and pass in argon gas. Cool to 0°C with stirring, add 6 mL (0.1 mol) of dimethyl sulfide, and white precipitate appears. Use carbon tetrachloride-dry ice-cooling to -25°C, add dropwise a solution of 6.24g (0.04mol) of the mixture of cis and trans isomers of 4-tert-butylcyclohexanol (2) dissolved in 40mL of toluene, and complete the addition in about 5 minutes. Continue to stir the reaction at -25°C for 2 hours, then add dropwise a solution of 6.0g (0.59mol) triethylamine dissolved in 10mL toluene, and complete the addition in about 3 minutes. Remove the cold bath and add 400 mL of diethyl ether after 5 minutes. The organic layer was washed with 100 mL (1%) hydrochloric acid and then 100 mL water. Dry over anhydrous magnesium sulfate, and evaporate the solvent under reduced pressure. The residue was transferred to a 50mL round-bottomed flask, distilled under reduced pressure, and the fraction at 120°C/3.325kPa was collected to obtain 5.54-5.72g of compound (1), with a yield of 90%-93%. Cures after standing, mp41~45℃. [3]
Purpose
Suitable for use as daily fragrance.

 微信扫一扫打赏
微信扫一扫打赏

