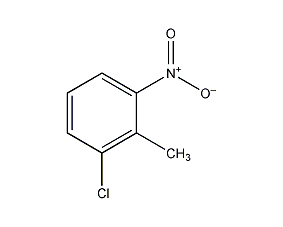
Structural formula
| Business number | 01TV |
|---|---|
| Molecular formula | C7H6ClNO2 |
| Molecular weight | 171.58 |
| label |
6-nitro-2-chlorotoluene, 2-Chloro-6-nittoluene, 1-Chloro-2-methyl-3-nitro-benzen, CH3C6H3(NO2)Cl |
Numbering system
CAS number:83-42-1
MDL number:MFCD00007205
EINECS number:201-475-9
RTECS number:XS9130000
BRN number:1239924
PubChem number:24855517
Physical property data
1. Characteristics: needle-like crystals.
2. Density (g/mL,25/4℃): Unsure
3. Relative vapor density (g/mL,AIR=1): Unsure
4. Melting point (ºC): 37 -40℃
5. Boiling point (ºC,Normal pressure):238℃
6. Boiling point (ºC,5.2kPa): Unsure
7. Refractive index:1.5377(69 ℃)
8. Flashpoint (ºC): 125℃
9. Specific optical rotation (º): Unsure
10. Autoignition point or ignition temperature (ºC): Unsure
11. Vapor pressure (kPa,25ºC): Unsure
12. Molar volume (m3 /mol):129.5
3. isotonic specific volume (90.2K):336.2
4. Surface Tension (dyne/cm):45.4
5. Polarizability(10-24cm3):16.85
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 45.8
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 157
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be kept sealed and dry.
Synthesis method
Obtained from the nitration of o-xylene. Cool o-xylene to -10℃, add pre-cooled mixed acid dropwise while stirring (1 portion66%nitric acid2 portion98%sulfuric acid). The dripping temperature is controlled at-10~-5℃, add, continue stirring0.5h. Leave to stand to separate the waste acid from the lower layer, take the nitrification solution from the upper layer and use water and water in sequence. 5%Sodium hydroxide solution and water washing, then steam distillation, and then fractionation to obtain3-Nitro-o-xylene. Yield50%.
Purpose
Used as pharmaceutical intermediates .
: 9pt; FONT-FAMILY: 宋体; mso-ascii-font-family: Arial; mso-hansi-font-family: Arial; mso-bidi-font-family: Arial; mso-ansi-language: EN-US; mso -fareast-language: ZH-CN; mso-bidi-language: AR-SA”>℃, add pre-cooled mixed acid dropwise under stirring (1 shares 66%nitric acid2 portion98%formed with sulfuric acid). The dripping temperature is controlled at-10~-5℃, add, continue stirring0.5h. Leave to stand to separate the waste acid from the lower layer, take the nitrification solution from the upper layer and use water and water in sequence. 5%Sodium hydroxide solution and water washing, then steam distillation, and then fractionation to obtain3-Nitro-o-xylene. Yield50%.
Purpose
Used as pharmaceutical intermediates .
ter: line-break”>
Purpose
Used as pharmaceutical intermediates .

 微信扫一扫打赏
微信扫一扫打赏

