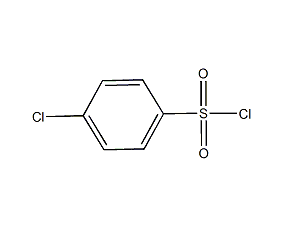
Structural formula
| Business number | 02E8 |
|---|---|
| Molecular formula | C6H4Cl2O2S |
| Molecular weight | 211 |
| label |
p-Chlorobenzene sulfonyl chloride, p-Chlorobenzenesulfonyl chloride, aromatic sulfur compounds |
Numbering system
CAS number:98-60-2
MDL number:MFCD00007439
EINECS number:202-685-3
RTECS number:DB8925000
BRN number:511583
PubChem number:24854131
Physical property data
1. Properties: White prismatic crystals.
2. Density (g/mL, 25℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 53
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 2kpa): 141
7. Refractive index: Undetermined
8. Flash point (ºC): 107
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 40ºC): Not determined
12. Saturated vapor pressure (kPa, 88ºC ): 0.13
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined Determined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: soluble in ethanol, insoluble in water. Decomposes when exposed to water.
Toxicological data
1. Skin/eye irritation: Standard Dresser test: rabbit skin contact, 20mg/24HREACTION SEVERITY, moderate reaction; Standard Dresser test: rabbit eye contact, 50μg/24HREACTION SEVERITY, strong reaction; 2. Acute toxicity: large Mouse oral LD50: 4250mg/kg; Mouse peritoneal cavity LDL0: 250mg/kg;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 45.22
2. Molar volume (cm3/mol): 138.0
3. Isotonic specific volume (90.2K ): 359.3
4. Surface tension (dyne/cm): 45.9
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 17.92
Computational ChemistryData
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 42.5
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 211
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with humid air, strong oxidants, and strong alkali.
Poisonous.
Storage method
1. Store in a cool, dry and well-ventilated warehouse. Keep away from fire, heat and water sources. The packaging must be sealed and protected from moisture. They should be stored separately from oxidants and alkalis, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
2. Packed in iron drums and stored in a cool, dry and ventilated place.
Synthesis method
1. The p-aminobenzene sulfonic acid method uses p-aminobenzene sulfonic acid as raw material. It undergoes diazotization and Sandmeyer reaction to generate p-chlorobenzenesulfonic acid. Add sodium hydroxide to generate sodium p-chlorobenzene sulfonate, and add chlorine to it. In sulfonic acid, react at 40-60°C for 3.5 hours. After cooling, drop into ice water, filter at 150°C, and wash to ph=5.5-6 to obtain the product. After recrystallization and purification, a high-purity product is obtained. Due to the long reaction route and high cost of the p-aminobenzenesulfonic acid method, currently no production units in our country have adopted it.
2. The chlorobenzene and chlorosulfonic acid method uses chlorobenzene and chlorosulfonic acid as raw materials. Cool the chlorosulfonic acid and sulfuric acid to below 20°C, add chlorobenzene under strong stirring, and stir at 23-25 React at ℃ for 2-3 hours, raise the temperature to 60°C, keep it for 2 hours, cool and filter to obtain the product, and obtain a high-purity product after recrystallization and purification. Raw material consumption quota: chlorosulfonic acid 2200kg/t, chlorobenzene 650kg/t, sulfuric acid 350kg/t.
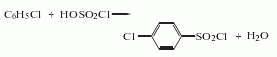
3. Its preparation method is: Prepared by chlorosulfonation of chlorobenzene. Mix chlorosulfonic acid and fuming sulfuric acid and cool to below 20°C. Add chlorobenzene under strong stirring and react at 23-25°C for 2-3 hours. Then raise the temperature to 60°C and react for 2 hours. Cool, filter and wash away. Derived from acidity. In addition, it can also be obtained by reacting p-chlorobenzenesulfonic acid with chlorosulfonic acid at 60°C for 1.5 hours.
Purpose
Mainly used in the preparation of pharmaceuticals, engineering plastics, pesticide intermediates and organic synthetic raw materials p-chlorobenzenesulfonamide. Used in the production of pesticides, acaricides, acaricides, pharmaceuticals, etc.

 微信扫一扫打赏
微信扫一扫打赏

