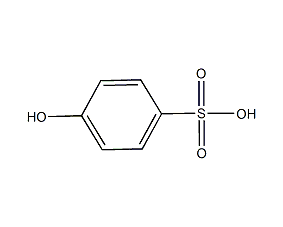
Structural formula
| Business number | 02EC |
|---|---|
| Molecular formula | C6H6O4S |
| Molecular weight | 174.17 |
| label |
Phenol-4-sulfonic acid, 4-Hydroxybenzenesulfonic acid, HOC6H4SO3H, p-Phenolsulfonic acid, Phenol-4-sulfonic acid, Phenolsulfonic acid |
Numbering system
CAS number:98-67-9
MDL number:MFCD00007506
EINECS number:None
RTECS number:DB6970000
BRN number:1869034
PubChem number:24850348
Physical property data
1. Properties: Yellow water-absorbing crystals, which can turn brown in the air.
2. Density (g/mL, 25℃): 1.337
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 50 (decomposition)
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 22mmHg): Undetermined
7. Refractive index: 1.489
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 40ºC): Not determined
12. Saturated vapor pressure ( kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/ V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in water.
Toxicological data
Acute toxicity: Mouse oral LD50: 6400mg/kg;
Ecological data
This substance is harmful to the environment and can cause pollution to water bodies and the atmosphere. Organic acids can easily form acid rain during atmospheric chemistry and atmospheric physical changes. Therefore, when the pH value drops below 5, it will cause serious harm to animals and plants, and the reproduction and development of fish will be seriously affected. Metals in the soil and water sediments in the watershed can be dissolved into the water and poison the fish. Acidification of water bodies will also lead to changes in the composition and structure of aquatic organisms. Acid-resistant algae and fungi will increase, while root plants, bacteria and vertebrates will decrease, and the decomposition rate of organic matter will decrease. Acidification will seriously lead to the reduction or death of fish in lakes and rivers.
Molecular structure data
1. Molar refractive index: 38.63
2. Molar volume (cm3/mol): 110.6
3, Isotonic specific volume (90.2K): 315.4
4. Surface tension (dyne/cm): 66.1
5. Dielectric constant:
6. Even Polar distance (10-24cm3):
7. Polarizability: 15.31
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 2
6. Topological molecule polar surface area 83
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 207
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidants and alkalis.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants and alkalis, and avoid mixed storage. Suitable materials should be available in the storage area to contain spills.
Synthesis method
None yet
Purpose
It is used for resin curing and is the most important additive in the acidic tin plating process. It also has the function of acidic resin foaming and is used as an organic intermediate.

 微信扫一扫打赏
微信扫一扫打赏

