Background and overview[1]
2-Fluoro-3-(trifluoromethyl)phenol is an organic fluoride that can be used as a pharmaceutical synthesis intermediate.
Preparation[1]
The preparation of 2-fluoro-3-(trifluoromethyl)phenol is as follows:
Under a nitrogen atmosphere, put compound (t-20) (24.5g, 149.30mmol) and THF (300mL) into a reaction vessel, and cool to -70°C or lower. Then, n-butyllithium (1.60 M; n-hexane solution; 111.97 mL, 179.16 mmol) was added dropwise thereto in a temperature range of -75°C to -70°C, and the resulting mixture was further stirred for 1 hour. A solution of trimethyl borate (18.62 g, 179.16 mmol) in THF (50 mL) was added dropwise thereto at a temperature ranging from -75°C to -70°C, and the resulting mixture was stirred for 3 hours while returning to room temperature. Acetic acid (12.82 mL, 223.95 mmol) was added dropwise to it in the temperature range of 20°C to 25°C. After 30 minutes, hydrogen peroxide (30% aqueous solution, 33.86g, 298.60mmol) was added dropwise to it. The resulting mixture was stirred for a further 10 hours at a temperature in the range of 25°C to 35°C. The resulting reaction mixture was poured into water and extracted with ethyl acetate. The combined organic layers were washed successively with water, sodium sulfite aqueous solution, water and saturated brine, and dried over anhydrous sodium sulfate. The resulting solution was concentrated under reduced pressure to obtain compound 2-fluoro-3-(trifluoromethyl)phenol (26.89g, 149.30mol; 99%). Compound 2-fluoro-3-(trifluoromethyl)phenol can be used in the next reaction without purification.
Apply[1]
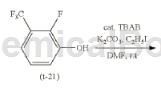

2-Fluoro-3-(trifluoromethyl)phenol can be used as a pharmaceutical synthesis intermediate. If the following reaction occurs:
Under nitrogen atmosphere, put 2-fluoro-3-(trifluoromethyl)phenol (26.89g, 149.30mmol) and THF (600mL) into the reaction vessel, and then cool to -70°C or lower. Next, lithium diisopropylamide (LDA) of THF solution (1.09M, 167.5mL, 182.6mmol) was added dropwise thereto at a temperature of -70°C or lower. The resulting mixture was stirred at -70°C or lower for 1 hour, and then N,N-dimethylformamide (DMF; 14.7g, 200.8mmol) in THF (30mL) was added dropwise thereto at -70°C. solution. Or lower. The resulting mixture was stirred at -70°C or lower for 2 hours, and then the resulting reaction mixture was poured into an ice-added aqueous ammonium chloride solution and stirred for 15 minutes. The obtained reaction liquid was separated into an organic layer and an aqueous layer, and the aqueous layer was extracted with ethyl acetate. The combined organic layers were washed with water and saturated brine, and dried over anhydrous sodium sulfate. The resulting solution was concentrated under reduced pressure, and the residue was purified by column chromatography (filler: silica gel, eluent: hexane/ethyl acetate = 4/1), and then recrystallized from heptane to obtain compound (t-22) (21.1g) , 101.37mmol, 67.9%).
Main reference materials
[1](US20150218452)Carbonylderivative, liquid crystal composition containing compound thereof and liquid crystal display device

 微信扫一扫打赏
微信扫一扫打赏

