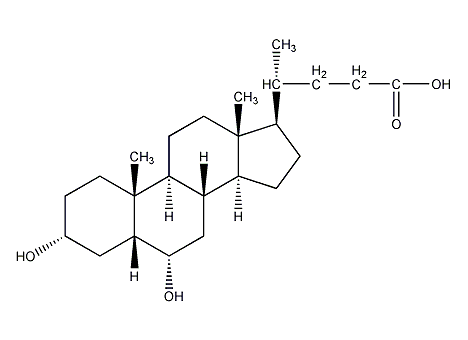
Structural formula
| Business number | 01U0 |
|---|---|
| Molecular formula | C24H40O4 |
| Molecular weight | 392.57 |
| label |
hyodeoxycholic acid, Isodeoxycholic acid; 3α, 6α-dihydroxy-5β-cholanic acid; α-hyodeoxycholic acid; , 3α,6α-Dihydroxy-5β-cholan-24-oic acid, HDCA, Lipoids |
Numbering system
CAS number:83-49-8
MDL number:MFCD00003681
EINECS number:201-483-2
RTECS number:FZ2050000
BRN number:None
PubChem number:24895601
Physical property data
1. Properties: white or slightly yellow crystal.
2. Density (g/mL, 25/4℃): Uncertain
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 196~197℃
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa) : Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (º): [α]D20 +8° (in ethanol).
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturation Vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (% , V/V): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: Soluble in ethanol and glacial acetic acid, slightly soluble In ether, acetone, ethyl acetate and benzene, almost insoluble in water.
Toxicological data
Teratogenicity
Yeast: 5600 umol/L
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 109.65
2. Molar volume (cm3/mol): 347.8
3. Isotonic specific volume (90.2K ): 905.9
4. Surface tension (dyne/cm): 46.0
5. Polarizability (10-24cm3): 43.46
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 4.9
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 4
5. Topological molecular polar surface area (TPSA): 77.8
6. Number of heavy atoms: 28
7. Surface charge: 0
8. Complexity: 605
9. Isotopes Number of atoms: 0
10, Determine the number of atomic stereocenters: 10
11, Uncertain number of atomic stereocenters: 0
12, Determine chemical bonds Number of stereocenters: 0
13, Number of uncertain chemical bond stereocenters: 0
14, Number of covalent bond units: 1
Properties and stability
It can inhibit the formation of cholic acid and dissolve fat, reduce blood cholesterol and triglycerides, and is suitable for type Ia or Ib hyperlipidemia and atherosclerosis. It can stimulate bile secretion and thin the bile without increasing the solid content; it can also accelerate the discharge of cholecystography contrast agent from the liver and help develop imaging. It can also promote intestinal lipolysis and fat-soluble vitamin absorption.
Storage method
This product should be sealed and stored in a cool, dry place.
Synthesis method
1.
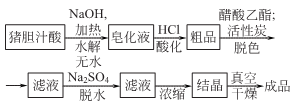
2.
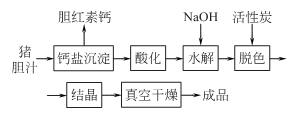
Add saturated lime water supernatant to fresh pig bile, heat to boiling to generate bilirubin calcium salt, filter, add hydrochloric acid to the mother liquor while it is hot, and acidify it until Congo red turns blue. Precipitate the black colloidal precipitate combined with bile acid, remove the emulsion, and collect the precipitate to obtain crude porcine bile acid. Take crude bile acid, add 1.5 times sodium hydroxide and 9 times water, and heat and hydrolyze it for more than 16 hours. After cooling, siphon off the upper light yellow liquid, and add an appropriate amount of water to the precipitate to dissolve it. Add hydrochloric acid or sulfuric acid to the aqueous solution to reach pH 3.0~3.5. Separate the precipitate, wash it with water until it is nearly neutral, and dry it in a vacuum to obtain crude porodeoxycholic acid. Take the crude porodeoxycholic acid and add 5 times the amount of ethyl acetate, then add 15% to 20% activated carbon, and heat to reflux to dissolve and decolorize. After cooling, filter, add 3 times the amount of ethyl acetate to the filter residue, reflux, and filter. Combine the filtrate and add 20% anhydrous sodium sulfate for dehydration. After filtering out sodium sulfate, the filtrate is concentrated to 1/5 to 1/3 of the original volume and allowed to cool for crystallization. The crystals are separated by filtration, washed with a small amount of ethyl acetate, and dried in a vacuum to obtain hyodeoxycholic acid.
Purpose
1. Used for biochemical research.
2.Suitable for type Ia and type Ib hyperlipidemia and atherosclerosis. It has certain antibacterial effects on Bacillus pertussis, Bacillus diphtheriae, Staphylococcus aureus, etc. It can be used as an anti-inflammatory drug to treat chronic bronchitis and viral upper respiratory tract inflammation in children. Suitable for cholangitis, cholecystitis, cholelithiasis and other non-obstructive cholestasis; it can accelerate the discharge of cholecystography contrast agent from the liver to facilitate visualization. It can also promote the decomposition of intestinal fat and the absorption of fat-soluble vitamins, and can be used for indigestion caused by liver and gallbladder diseases.

 微信扫一扫打赏
微信扫一扫打赏

