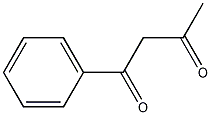
Structural formula
| Business number | 026X |
|---|---|
| Molecular formula | C10H10O2 |
| Molecular weight | 162.19 |
| label |
1-Phenylbutanedione-[1,3],α-acetylacetophenone, benzoyl acetone, Acetylbenzoylmethane, α-acetyl acetophenone, 1-Benzoylacetone, 1-Benzoyl-2-propanone, Benzoylacetone, C6H5COCH2COCH3 |
Numbering system
CAS number:93-91-4
MDL number:MFCD00008786
EINECS number:202-286-4
RTECS number:EK3540200
BRN number:742413
PubChem number:24847841
Physical property data
1. Properties: Colorless crystals.
2. Refractive index at room temperature (n25): 1.567878
3. Relative density (25℃, 4℃ ): 1.059974
4. Melting point (ºC): 60
5. Boiling point (ºC, normal pressure): 261.5
6. Boiling point (ºC, 5.2kPa): Not determined
7. Refractive index: 1.56775
8. Flash point (ºC): Not determined
9. Specific rotation (º): Not determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined Determined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical Temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
p>
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Easily soluble in Ethanol, ether and concentrated alkali solution, slightly soluble in cold water.
Toxicological data
Acute toxicity:
Oral LD50 >500mg/kg (rat)
Main irritant effects:
On skin: May cause inflammation.
In eyes: May cause irritation
Sensitization: No known sensitizing effects.
Ecological data
General Notes
Generally speaking, it is not harmful to water.
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 45.57
2. Molar volume (cm3/mol): 151.0
3. Isotonic specific volume (90.2K ): 377.0
4. Surface tension (dyne/cm): 38.7
5. Polarizability (10-24cm3): 18.06
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 5
6. Topological molecule polar surface area 34.1
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 178
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be kept sealed.
Synthesis method
1. Reaction of acetophenone and ethyl acetate. Add 6g of sodium ethoxide to 20g of ethyl acetate under cooling, and then add 10g of acetophenone after 15 minutes. When benzoyl acetone begins to precipitate, add a small amount of anhydrous ether, filter after 4 hours, wash with ether, and dry. Then it was dissolved in water and acidified with acetic acid to isolate 10 g of benzoyl acetone. 2. Reaction of acetophenone and acetic anhydride. Add 20g acetophenone and 34g acetic anhydride and add sodium acetate under cooling (45g sodium acetate is dissolved in 100ml water). Then use boron trifluoride to make it a saturated solution. Distilled with water vapor, it started to be an oily substance. After cooling, crystals precipitated. It was extracted with diethyl ether, dried and then distilled to obtain 22.5g of the product. 3. React benzoyl chloride and ethyl acetoacetate in the solvent ether, react benzoyl chloride with sodium ethyl acetoacetate to obtain benzoyl acetoacetate, then add water and reflux for 5 hours, and distill it with water vapor to obtain benzoyl acetone.
2. Preparation method:
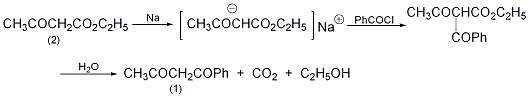
In a reaction bottle equipped with a stirrer, dropping funnel, and reflux condenser (with a calcium chloride drying tube on the top), add 300 mL of anhydrous ether and 5.6 g (0.23 mol) of metallic sodium wire, and cool slowly Slowly add 30g (0.23mol) of ethyl acetoacetate (2) dropwise, control the dropping speed, do not make the reaction too violent, and do not boil the ether. After adding, leave it overnight to form a white gel-like precipitate. While stirring, slowly add dropwise a solution of 32.4g (0.23mol) benzoyl chloride dissolved in 300mL anhydrous ether. After adding, leave it for 5 hours. Slowly add an appropriate amount of water to dissolve the resulting precipitate solution. The ether layer was separated and dried over anhydrous sodium sulfate. The ether was evaporated to obtain ethyl benzoyl acetoacetate as a dark yellow oil. The above oil obtained was refluxed with water for 5 hours, and then steam distilled. What initially came out was mainly acetophenone and a small amount of benzoyl acetone. When the distillate cools and begins to crystallize, replace the receiving bottle. Be careful not to condense the product in the condenser. After distillation, the distillate is cooled and filtered. Dissolve the solid in 1% sodium hydroxide solution, add carbon dioxide to saturation while cooling with ice water, and precipitate white needle-like crystalline benzoyl acetone. Filter and dry to obtain (1) 10g, mp58~60℃, yield 31%. [1]
Purpose
A reagent for the determination of thallium. Organic Synthesis.

 微信扫一扫打赏
微信扫一扫打赏

